
Important:
the immediate use of beta-blockers is contraindicated in pts with hypotension, active bronchospasm, heart block, severe bradycardia (generally, <40 beats per minute), or overt heart failure.


TIMI 2 flow (partial reperfusion) is delayed or sluggish antegrade flow with complete filling of the distal territory. TIMI 3 is normal flow which fills the distal coronary bed completely.
Troponin is a complex of 3 specific proteins found in striated muscle. 2 of the subunits, cardiac troponin T (cTnt) and I (cTni), are useful as clinical markers of myocardial injury. Because these cardiac proteins are genetically unique, cTnt & cTni are the most cardiac-specific biochemical markers. After an AMI, cardiac troponin serum levels generally elevate within 2–6 hrs, peak at 12–24 hrs, & may stay elevated for 7–10 days. The sensitivities of elevations of cTnt & cTni between 2 - 6 hrs of an AMI are 59–90% & 69–82%, respectively. However, both assays approach 100% sensitivity between 6 -12 hrs after admission. The specificities do not vary significantly over time with the of elevations of cTnt & cTni of 93–98% and 93–99%, respectively. Newer high-sensitivity troponins have not yet been approved for use in the US but have shown increase in sensitivity for Dx of infarction at the cost of specificity.
Creatinine kinase muscle band (CK-MB) is found not only in the myocardium but also in brain and skeletal muscle; it is less specific for a myocardial ischemic event than some other markers. Many clinicians do not order CK-MB levels any longer because of the superior sensitivity and specificity of the cardiac troponin marker. The 2014 American Heart Association (AHA) guidelines for NSTEMI state that CK-MB & myoglobin are no longer useful for the Dx of ACS.
A. Immediate Measures
Upon arrival, place pt on a cardiac monitor, give oxygen if O2 saturation is < 90% & place 2 peripheral IVs. Aspirin is the first & most important med given to ACS pts early in their course. Give 325 mg nonenteric-coated chewable aspirin. If contraindicated, use 300 mg clopidogrel. Coumadin use is not a contraindication to single aspirin dose, & does not substitute for aspirin as the mechanism of action is different. NSAIDs should not be used to treat chest pain thought to be cardiac in origin.
If chest pain is present, give nitroglycerin, 0.4 mg sublingually or one spray delivered to the oral mucosa. Repeat if no effect occurs in 5 mins. If chest pain returns or continues & SBP > 100 mm Hg, start IV nitroglycerin at 10 μg/min & increase by 5 μg/min every 3–5 minutes until SBP falls by 10% or chest pain is relieved. The SBP should not drop below 90 mm Hg. Morphine may be still be used for analgesia, particularly for STEMI pts. However, for unstable angina/NSTEMI pts, it may have increased adverse effects.
Current AHA guidelines recommend starting oral β-blockers within 24 hrs of presentation. IV β-blockers may be considered for refractory HTN or ongoing ischemia. Contraindications to giving β-blockers include CHF, bradycardia, conduction blocks, hypotension. Also, be aware of higher risks if given in elderly pts with suspected cocaine use & COPD/asthma pts.
B. Additional Measures
Establish a lab test database including CBC with differential, serum creatinine & electrolyte measurements, BUN, & enzyme levels (cTni or cTnt). Platelet count, prothrombin time, partial thromboplastin time, & blood for typing (& cross-matching if needed) should be obtained for pts to be given thrombolytic therapy.
C. Treatment of STEMI
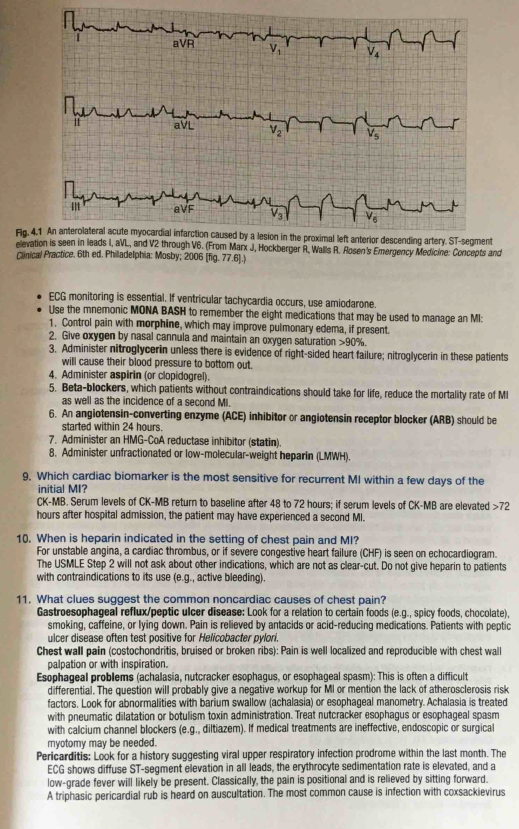
The definition of STEMI is new ST elevation at the J point in at least 2 contiguous leads of >= 2 mm (0.2 mV) in men or >= 1.5 mm (0.15 mV) in women in leads V2–V3 and/or >= 1 mm (0.1 mV) in other contiguous chest leads or the limb leads. The 2 methods currently available are pharmacologic thrombolysis via plasminogen activators & percutaneous coronary intervention (PCI). The effectiveness of either modality in reducing mortality & myocardial damage depends on how early it is given after the onset of symptoms.
Current guidelines recommend thrombolytic administration for STEMI in the absence of contraindications when onset of symptoms is within the previous 12 hours & PCI cannot be performed within 120 minutes of first medical contact. Thrombolytics should be initiated in the ED because the benefit of pharmacologic thrombolysis decreases with each passing hour after MI. There is less evidence for thrombolytics with onset of symptoms from 12 - 24 hrs but current recommendations suggest it may be a reasonable choice for ongoing ischemia when a large area of myocardium is at risk or there is hemodynamic instability.
Many thrombolytic agents are available, including streptokinase (no longer available in the US), alteplase (tPA), reteplase (rPA), tenecteplase (TNK). Alteplase administration over 90 mins improved survival when compared to streptokinase or 3-hour alteplase infusion. Even though intracranial bleeding events increased, alteplase demonstrated a long-term survival advantage presumably secondary to earlier thrombolysis & reperfusion of thrombosed coronary arteries. Compared to alteplase, TNK may offer advantages of a single bolus administration and fewer intracranial hemorrhage complications. Intracranial hemorrhage is the most devastating complication of thrombolytic therapy, occurring in 0.5–3.3% of pts.
Absolute contraindications are as follows:
History of any intracranial hemorrhage
Any known structural cerebral vascular lesion
Significant closed-head or facial trauma within 3 months
Intracranial or intraspinal surgery within 2 months
Any ischemic stroke within 3 months
Any intracranial neoplasm
Active internal bleeding (eg, serious gastrointestinal bleeding) excluding menses
Suspected aortic dissection
Uncontrolled hypertension that is severe and refractory to emergency treatment
Streptokinase: Previous treatment within 6 months
In the following conditions, the risks associated with thrombolytic therapy may be increased, use clinical judgment in evaluating expected benefits:
Recent (within 10 days) puncture of a noncompressible blood vessel
Poorly controlled HTN of several years’ duration
Significant hypertension on presentation (DBP >110 mm Hg or SBP >180 mm Hg)
Diabetic hemorrhagic retinopathy or hemorrhagic ophthalmic condition
Oral anticoagulant therapy
Major surgery within 3 wks
Pregnancy, active peptic ulcer, dementia
Internal bleeding within 2–4 wks
Traumatic or prolonged (>10 minutes) CPR
History of non-hemorrhagic cerebrovascular accident beyond 3 months
Admit pts given thrombolytic therapy to an ICU as soon as possible after initiation of treatment. Monitor the following:
BP every 15 mins during infusion & every 30–60 mins thereafter
ECG rhythm strip for reperfusion arrhythmias & ST-segment changes
Bleeding complications & changes in neurologic status; avoid venous or arterial punctures & unnecessary trauma
12-Lead ECG 4 hrs after the start of therapy & as needed for changes
cTni or cTnt 4 hours after initiation of treatment & at 4-hour intervals for 24 hours
All pts should receive aspirin as mentioned above.
Oral Antiplatelet therapy
Pts undergoing PCI may receive clopidogrel, prasugrel (if no contraindications) or ticagrelor.
Pts undergoing fibrinolysis should receive clopidogrel 300 mg for patients < 75 years or 75 mg for those > 75.
Anticoagulation
Pts undergoing PCI may receive heparin or bivalirudin.
Pts undergoing fibrinolysis may receive heparin or another anticoagulant such as enoxaparin, or fondaparinux. IV heparin should be given in a separate line while alteplase is infusing because of the short half-life of alteplase & the danger of recurring thrombosis. Anticoagulation should be continued for 48 hrs or longer.
Glycoprotein (GP) IIb/IIIa receptor antagonists
Some PCI centers may use these medications in selected pts.
Give oxygen if indicated. Hospitalize pt & obtain serial ECGs & myocardial enzyme determinations (cTni or cTnt) to detect possible MI.
Start nonenteric aspirin, 325 mg chewable.
Nitroglycerin paste, sublingual tablets, or spray may all be considered. If the pain persists, an IV infusion of nitroglycerin is indicated at an initial rate of 10 μg/min.
Add a β-adrenergic blocking agent (eg, metoprolol, 25 mg orally) unless contraindicated (HR < 60 beats/min, atrioventricular block, severe asthma, or COPD). β-Blocker use is recommended in first 24 hrs of unstable angina.
Morphine’s use in cardiac chest pain, particularly NSTEMI has been diminished as some observational studies showed worsened outcomes. However, it may be considered in cases of refractory or severe pain. Do not use NSAIDS for suspected cardiac chest pain as they have been shown to increase mortality.
Clopidogrel or ticagrelor may be given. Ticagrelor is the first oral P2Y12 receptor antagonist that blocks ADP-induced platelet aggregation in a reversible manner.
Dual antiplatelet treatment with aspirin & clopidogrel is a cornerstone of therapy for pts with acute MI whether treated invasively or noninvasively. Old??
Unlike clopidogrel & prasugrel, ticagrelor is an orally active antagonist that reversibly binds to the P2Y12 platelet receptor, which yields faster, greater &more consistent inhibition of platelet aggregation.
Anticoagulation may also be helpful in these patients. Use unfractionated heparin at 60–70 units/kg intravenous bolus with a maximum of 5000 units followed by 12 units/kg/h intravenous infusion or a low-molecular-weight heparin such as enoxaparin, 1 mg/kg administered subcutaneously every 12 hours. Other agents available include bivalirudin and fondaparinux.
Glycoprotein IIB/IIIA receptor antagonists such as abciximab and the newer agents tirofiban and eptifibatide are approved for use in patients with ACS. These agents block platelet binding at the receptor site that cross-links fibrinogen to the platelets. The IIb/IIIA inhibitors are often added on top of above medications in more high-risk patients if catheterization or PCI is planned.
If pain continues despite treatment, consider PCI. If AMI has occurred and pain continues despite optimal medical management or thrombolytics, PCI must be considered.
Hospitalize all pts with clinical histories suggesting MI. For STEMI pts, ideally, a hospital capable of performing PCI will have a mechanism in place that allows an emergency physician to directly activate the cardiac catheterization lab so as to get the pt to definitive treatment as rapidly as possible. Pts with suspected MI & normal initial ECGs & initial cardiac enzymes may be admitted to a monitored intermediate care unit.
About 10–15% of pts reaching the hospital with MI die during hospitalization. One or more complications occur in over half of all MI pts.
Shock complicating MI occurs in 5–10% of pts and may be caused by extensive MI with decreased cardiac output (most common), inappropriate reflex peripheral vasodilatation, arrhythmias, hypovolemia, RV infarction, & mechanical complications such as ruptured ventricular septum & severe mitral regurgitation. Free-wall myocardial rupture results in tamponade & shock. The mortality rate is as high as 70–80% among pts with cardiogenic shock as a complication of AMI & is the leading cause of death in pts hospitalized with MI.
Hypotension accompanied by confusion, obtundation or restlessness, cool skin, oliguria, & metabolic acidosis suggests shock. Mild-to-moderate hypotension alone is common in MI & does not itself indicate shock. Shock in MI may be due to many causes (see Table 11–1), which may be difficult to differentiate (Table 34–1).
Table 34–1.Differential diagnosis by hemodynamics of heart failure and hypotension after myocardial infarction.View Table|
Use any or all of the measures discussed here as necessary (see also Chapter 11).
Give oxygen by mask or nasal cannula. Pts in shock with respiratory failure require endotracheal intubation.
Consider monitoring central pressure with a Swan-Ganz pulmonary artery catheter (or, far less desirably, a central venous pressure catheter, since in acute MI, left ventricular filling pressure can be markedly elevated with normal right ventricular filling pressure, & vice versa). Use an arterial line to measure BP.
Give a fluid challenge (200 mL of saline IV over 20 mins) if the pt is not in CHF (ie, no rales & no pulmonary edema on CXR). Repeat as needed if CHF does not develop. Correct arrhythmias (see below). Insert a Foley catheter, & measure urine output hourly.
The need for inotropic or pressor support with meds such as dobutamine, dopamine, and norepinephrine should be assessed on an individual basis. Use the smallest effective dose, guided by hemodynamic response.
Evidence indicates that acute revascularization by PCI decreases mortality rates compared to immediate medical stabilization. PCI acutely should be seriously considered in such pts because thrombolytics are ineffective in cardiogenic shock associated with an acute MI. An intra-aortic balloon pump (IABP) can also be placed, typically in the catheter lab, but again this therapy should be considered on a pt by pt basis. IABP counterpulsation can increase cardiac output & improve both coronary & systemic perfusion. IABP counterpulsation is contraindicated in pts with aortic valve dz or those with aortic dissection.
All pts with cardiogenic shock must be hospitalized, preferably in an ICU. Therapy is directed at the likely causes of shock.
CHF is caused by extensive MI, volume overload, arrhythmias, acute mitral regurgitation, or ventricular septal rupture.
Symptoms & signs of CHF include dyspnea, anxiety, tachypnea, tachycardia, pulmonary rales or frank pulmonary edema, jugular venous distention, hypoxemia, & typical findings on CXR (cardiomegaly, pulmonary vascular plethora, Kerley B lines, pleural effusion, or pulmonary infiltrates consistent with pulmonary edema). Wheezing may also be a sign of CHF (cardiac asthma). Suspect RV infarction in inferior MI if signs of R heart failure (RV gallops, elevated central venous pressure, hepatomegaly, peripheral edema) are prominent in the absence of signs of L heart failure (dyspnea, rales, pulmonary congestion on CXR).
Give oxygen if indicated. Treat respiratory failure if present.
Give NTG either sublingual or spray, followed by IV infusion. NTG will decrease preload as well as afterload. In pts with inferior or right ventricular AMI, nitrates are relatively contraindicated because they may precipitate profound hypotension. Hypotension related to nitrates is treated with reduction or discontinuation of the infusion depending on the degree of symptomatic hypotension. Intravascular volume expansion with IV fluid infusions will often quickly correct hypotension.
Give furosemide as an IV bolus of at least the pt's normal total daily dose. If the pt is not already taking furosemide, administer a 40-mg IV bolus initially, & observe the diuretic response by monitoring the pt's symptoms & urine output. Diuretics are contraindicated if right ventricular infarction is suspected.
can be used to decrease afterload.
has been shown to decrease need for intubation. Alveoli are kept open with positive pressure & work of the heart is reduced.
In the setting of an AMI, pts with CHF should be closely monitored in an ICU.
Mechanical failure of infarcted tissue (eg, rupture of the ventricular septum or of papillary muscle supporting the chordae tendineae) is a common cause of acute mitral regurgitation & ventricular septal rupture. Minimal-to-moderate mitral regurgitation is common after MI as a result of papillary muscle & left ventricular wall dysfunction. Severe degrees of mitral regurgitation can result from marked ischemia with little or no infarction & can be completely reversed with revascularization.
Abrupt, severe CHF with pansystolic regurgitation murmur suggests acute mitral regurgitation or ventricular septal rupture. Echocardiography to detect mitral regurgitation or the abnormal velocity jet of a ventricular septal defect can establish the Dx.
Treat heart failure with diuretics, morphine, & nitroglycerin. Obtain urgent cardiologic & cardiac surgical consultation. IABP is a useful temporizing measure while the pt is being prepared for surgery.
The only lifesaving treatment for most pts is emergency cardiac catheterization followed by surgery.
Disposition: Hospitalize all pts in a critical care unit for treatment & surgery.
The chief cause of myocardial rupture is mechanical failure of an infarcted ventricular wall.
Myocardial rupture is an uncommon cause of sudden death during acute MI; it is responsible for only about 5% of deaths. Myocardial rupture is suggested by abrupt onset of hypotension with increased venous pressure (ie, cardiac tamponade). Pulseless electrical activity often occurs.
If echocardiography or bedside emergency ultrasound demonstrates a pericardial effusion, pericardiocentesis is indicated & can be performed under ultrasound guidance. When emergent ultrasound is not available to assess a pt for possible pericardial tamponade, blind pericardiocentesis may be lifesaving.
Obtain emergency cardiac surgical consultation for immediate cardiac surgery. This is successful in the few cases in which rupture has been minimal with slow intrapericardial hemorrhage.
Systemic or pulmonary embolization is commonly caused by intracardiac mural thrombosis or phlebothrombosis.
The most common findings in pulmonary embolism are sudden unexplained dyspnea & tachycardia. Occasionally, pleuritic pain, signs of right heart strain, or abnormal chest X-ray may occur. Pts at greatest risk are those with thrombus visualized in the left or right ventricle by two-dimensional echo. The Dx may be confirmed by CT scan of the chest, lung scan, or arteriography. Systemic embolization is suspected when Sxs & signs of arterial occlusion occur. The clinical picture depends on the artery occluded, for example, flank pain & hematuria with renal artery embolism; pallor, pain, & loss of pulse with brachial or femoral artery embolism; stroke with cerebral artery embolism.
Give oxygen, draw blood for determination of prothrombin & partial thromboplastin times, and then begin systemic anticoagulation with heparin, 80 units/kg bolus followed by 18 units/kg/h infusion. Pericarditis is a relative contraindication to anticoagulation because of the risk of bleeding into the pericardial sac, with resulting cardiac tamponade. Heparin is also contraindicated in pts with recent stroke, active duodenal ulcer, or active bleeding that cannot be controlled by direct pressure. With a massive pulmonary embolism with right heart failure or shock, IV fibrinolysis with tPA has been recommended (see Chapter 33).
Seek appropriate surgical consultation (with a thoracic, general, or vascular surgeon) for pts with persistent hypotension, contraindications to thrombolytic therapy, or systemic embolization who may benefit from surgical intervention (eg, angioplasty & embolectomy for a pulseless & ischemic extremity).
Pts who have received tPA or have hemodynamic instability should be hospitalized in an ICU.
When transmural MI causes pericardial inflammation over the area of necrosis, pericarditis may occur within the first week. Pericarditis occurring more than 1 wk after MI may be the result of Dressler syndrome, an autoimmune reaction.
Pericarditis usually does not appear until 2–3 days after the onset of MI. The appearance of a friction rub is often the only manifestation; pain & ECG changes are often absent. Frequently, a small pericardial effusion may be detected by echocardiography. If a pericardial friction rub is heard in the first 24 hours after onset, suspect pericarditis as a primary Dx rather than as being due to acute MI. Early ECG signs of pericarditis include diffuse ST-segment elevation (with a concave upslope & indistinct J point) & diffuse PR-segment depression (except in lead aVR, where the PR segment is elevated).
Aspirin is recommended by the ACCF/AHA guidelines for treating post-STEMI pericarditis. Colchicine, acetaminophen, or narcotics may also be considered if aspirin does not control the pain.
Hospitalize the pt for pain control & monitoring for possible cardiac tamponade (rare).
Source:
Nitroglycerin is an appropriate treatment choice. The combination of elevated blood pressure and chest pain suggests an acute coronary syndrome. Nitroglycerin, which acts primarily by dilating veins and reducing preload, thereby reducing ventricular wall stress and oxygen demand, could help to relieve the patient’s chest pain and reduce his blood pressure, thereby reducing further myocardial damage.
Nitroglycerin is contraindicated in patients with hypotension, which is generally defined as a systolic blood pressure of less than 90 mm Hg. Hypotension could suggest right ventricular infarction, in which right ventricular filling and cardiac output are dependent on preload. In this patient, inferior myocardial infarction cannot be ruled out, but his high blood pressure makes it unlikely, and the benefits of administration outweigh the risks.
The use of nitroglycerin may also be contraindicated in patients who have taken a phosphodiesterase inhibitor (e.g., sildenafil) within the preceding 24 to 48 hours. If light-headedness or hypotension develops after the administration of nitroglycerin, the patient should quickly be lain flat, with his legs elevated, to increase preload.
In general, the administration of beta-blockers within the first 24 hours after MI has multiple benefits, including a reduced risk of death. The lowering of the heart rate & the force of muscular contractions reduces myocardial O2 demand.
In a health care setting, O2 should be administered in a pt with a suspected acute coronary syndrome if the oxygen saturation is < 94%. The goal would be to increase it to a maximum of 94%, since hyperoxia may have deleterious effects.
At an altitude of 32,000 feet, ~ 50% of passengers will have an O2 saturation of < 94%. Unfortunately, pulse oximeters are not available in the standard emergency medical kit. Oxygen can be delivered at a low rate of flow (2 liters per minute) or a high rate of flow (4 liters per minute). Passengers who know they will need supplemental oxygen during air travel — owing to long-term use or to a history of exacerbated hypoxia at cruising altitude — are required to request a portable oxygen concentrator from the airline 48 to 72 hours before their flight.
Loop diuretics, such as the furosemide or bumetanide provided in the medical kit, should be considered if there are symptoms or signs of volume overload (e.g., SOB with elevated jugular venous pressure, rales on pulmonary auscultation, or peripheral edema) & if the BP is normal or elevated. Similar findings in a pt with BP that is low or borderline may signal cardiogenic shock, in which case diuretics should be withheld.
There is no indication that this pt has volume overload. Since he is hypertensive, furosemide could be used to reduce his BP, but nitroglycerin or another antihypertensive medication would be preferred. In addition, if there is concern for inferior MI, a preload-dependent state, loop diuretics could precipitate hypotension.
The administration of IV fluid bolus of 500 mL can be an inappropriate treatment choice.
Comments
The pt's blood pressure is elevated, & although he shows no clinical signs of heart failure, there is no indication for the administration of IV saline. Consider giving IV fluids to pts with acute coronary syndrome if hypotension is present, a right ventricular infarction is suspected, or both. In such cases, increasing preload may improve cardiovascular function.
Take-home Points
New RBBB associated with acute MI often suggests a proximal LAD lesion.
In patients with LMT obstruction, there are 2 prominent electrocardiographic presentations: NSTEMI and STEMI.
The STEMI pattern plus RBBB and LAFB with an absence of ST-segment elevation in lead V1 indicates acute total occlusion of LMT without collateral circulation.
The ST-segment elevation in lead V1 indicates isolated proximal LAD occlusion, whereas its absence supports acute total occlusion of the LMT without collateral circulation.
In the early stage of acute MI, we should pay more attention to this ECG pattern in this very high-risk population, which can help reduce the mortality.
Source:
Chang Q, Jin Y. Acute Myocardial Infarction With Wide Complex Rhythm: What Is the Culprit Artery? JAMA Intern Med. Published online March 14, 2022. doi:10.1001/jamainternmed.2022.0122
Type II MI
Type 2 MI is defined as "myocardial infarction secondary to ischemia due to either increased O2 demand or decreased supply, e.g. coronary artery spasm, coronary embolism, anemia, arrhythmias, hypertension or hypotension."
The clinical problem remains: to distinguish acute MI (associated with plaque rupture or erosion, traditional or type 1 MI)—for which there is evidence-based treatment—from acute secondary ischemic cardiac injury, including type 2 MI, where such treatments are not evidence based & may even be harmful.
The role of troponin measurement is in the differential diagnosis of suspected NSTEMI. Pts presenting with STEMI require immediate intervention based on current guidelines & thus should not wait for troponin results.
The first stage is to assess the probability that the pt has underlying acute coronary artery disease (CAD) from the clinical hx & that the symptoms are caused by cardiac ischemia. The assessment of the probability that the pt has underlying CAD may be an informal classification into high, medium, or low risk or a more formal risk score. Cardiac ischemia is assessed by the ECG & interpretation of the symptoms. An initial troponin measurement should be made as well as assessment of renal function & other appropriate labs & investigations guided by the clinical presentation of the pt.
A holistic assessment is required at this point. This must assess the totality of the findings in a circular rather than linear fashion. It is important that the results of laboratory tests taken as a whole are consistent with the clinical features and the ECG. If the troponin is not elevated, then repeat testing is required before acute myocardial injury can be excluded with certainty. On the other hand, a single elevated troponin is not, on its own, diagnostic of MI. However, an elevated troponin along with other appropriate clinical and laboratory evidence raises the probability that the Dx is NSTEMI. The higher the troponin value, the greater the probability that the final diagnosis will be MI. It must be stressed that the data must be consistent. An elevated troponin plus a normal ECG or nonspecific changes should immediately raise suspicion of an alternate diagnosis. Similarly, there should be no other occult but acknowledged causes of minor troponin elevation, such as renal failure or significant age. Other potential acute causes of troponin elevation associated with CAD such as tachycardia or other conditions causing increased oxygen demand or reduced oxygen supply indicate a type 2 MI. However, even if the clinical probability of underlying CAD is high, a final Dx of MI requires demonstration of a changing troponin value. Contemporary sensitive assays are able to detect a significant troponin change on retesting if the initial value is elevated with a repeat test performed 2-3 hours later & with high sensitivity assays within 1 hour.
Repeat testing allows the immediate distinction between an acute myocardial injury & underlying chronic myocardial damage causing troponin elevation. This resolves the dilemma of the pt with renal disease (or high age). An elevated troponin in the 1st sample is to be expected in the pt with renal failure. A changing troponin indicates acute injury and may be due to an acute MI. In contrast, an elevated troponin that does not change significantly is due to chronic myocardial injury & may require further investigation but possibly not as an in-patient & not necessarily by a cardiologist.
The next phase of the evaluation is to distinguish between an acute troponin rise that is consistent with acute MI & one that is due to another cause. The Dx of acute MI should never be made on the basis of a troponin elevation alone. Again, a complete evaluation of the pt requires that the troponin rise is consistent with the clinical findings & the ECG. An elevated troponin with minimal ECG findings can occur with myocarditis or pulmonary embolism. The most important factor is to be aware that other clinical conditions can cause a troponin elevation. Troponin elevation is specific for myocardial injury, but not every troponin elevation is an MI. The presence of other clinical conditions such as pneumonia or pulmonary embolus should shift the clinical focus to an appreciation that the troponin elevation is an additional prognostic rather than diagnostic finding. If there is diagnostic uncertainty, cardiac imaging, either invasive or noninvasive, as well as other types of cross sectional imaging is necessary to provide additional information.
Therefore, the key features to diagnose a type 2 MI (more properly secondary ischemic cardiac injury), can be summarized as follows:
Treatment of type 2 MI is to treat the underlying condition & hence remove the cardiac insult. To adequately assess the prognosis & determine appropriate further treatment in pts with type 2 MI, information about whether the pt has (or is likely to have) significant underlying CAD is essential. In addition, remember that elevated troponin in the pt with non-acute MI is not redundant information but also indicates an adverse prognosis (Figure 1).

References