
Renal misinterpretation of decreased cardiac output as volume depletion leads to fluid retention and consequently acute decompensated heart failure (ADHF). This volume overload causes pulmonary congestion, abdominal bloating, and gravity dependent edema in the lower extremities or sacral region. While the pathophysiology of heart failure (HF) is diverse, the phenomena of ADHF occurs in both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). Although diuresis has not been shown to reduce long term mortality, removing excess fluid is necessary to reverse the pulmonary edema of ADHF that leads to shortness of breath and hypoxia (Ellison and Felker 2017).
*NOTE: The discussion below pertains to HFrEF (ejection fraction <40%).
Asking the correct questions is the key to unlocking an acute heart failure diagnosis. There are innumerable reasons for a patient to be short of breath, but orthopnea, shortness of breath while laying flat, is more specific for ADHF –though the LR is only 2.2 (Wang et al 2005). Uncover a history of orthopnea by asking not just how many pillows your patient uses, but also why your patient is using multiple pillows. Additionally, instead of just asking if a patient ever wakes up in the middle of the night, clarify if when they wake up do they feel short of breath or if waking up was due to other factors. Ask about abdominal bloating and lower extremity edema, patients often know how their ADHF presents. A history of HF is a great predictor of ADHF (Wang et al 2005).
Understanding the cause of ADHF is an important component of treatment; either you can address the underlying cause of an exacerbation, or an unprovoked decompensation suggests severe disease. It is important to take a history in a non-judgemental manner. Patients may be unaware of the salt content of food that they eat in a restaurant. The FAILURES mnemonic is a great way for remembering the factors that can precipitate ADHF:
Forgetting medication (or taking beta blockers, NSAIDs, methamphetamine, or cocaine)
Arrhythmia or Anemia
Ischemia or Infarction
Lifestyle choices including dietary indiscretions.
Upregulation of cardiac demand from either pregnancy or hyperthyroidism.
Renal failure from progression of kidney disease or insufficient dialysis.
Embolus (pulmonary embolism)
Stenosis from worsening renal artery stenosis, aortic stenosis, or other valvular disease.
While the evidence of sodium restriction in hypertension is strong, the role of sodium restriction (2-3 g daily) in HF is less conclusive (HFSA Guidelines 2010). However, it is important to ask your patient what foods, high sodium or high carbohydrate, lead to fluid retention for them. For more information on this topic, our friends at the Core IM podcast have a great episode on salt restriction and HF. A 2 liter fluid restriction is “recommended” by the HFSA Guidelines 2010 for those with severe hyponatremia (sodium <130 mEq/L) and can be “considered” for those with difficult to control fluid retention despite other measures (expert consensus).
The physical exam is crucial to diagnosing ADHF and risk stratifying patients. The two profiles of ADHF are warm-and-wet or cold-and-wet; warm-and-wet refers to well perfused decompensated patients, and cold-and-wet refers to sicker patients who may need inotropic support.
The jugular venous pressure (JVP) is estimated by observing the vertical height of the jugular venous pulse above the sternal angle, >4 cm is considered abnormal. This pulse is the internal jugular vein that can be found in the triangle formed by the sternocleidomastoid and the clavicle. To assess the JVP, recline the patient at 30 or 40 degrees with their head turned gently away from you, so as to not tamponade the vein with muscle or skin tension. Become one with the neck, and observe the biphasic flicker; the a wave corresponds with atrial contraction, and the v wave is from increased venous pressure during filling. If you see a c wave, theoretically formed by the bulging of the tricuspid valve during ventricular contraction, tweet at Dr. Kittleson, and she will be very impressed and/or not believe you. If the JVP is not visible, adjust the angle of the patient to bring it into view. To distinguish between carotid and jugular pulses, press on the belly to see if increased venous return will increase the presumably venous pulsation, or press on the pulsation itself, an arterial pulse will not compress but a venous pressure will. Elevated JVP is 11% sensitive and 97% specific for ADHF, and the addition of checking for the abdominojugular reflux increases sensitivity to 24% (Wang et al 2005).
Listen for an S3 gallop. S3 is 13% sensitive and 99% specific for heart failure; LR = 11; 95% CI, 4.9-25.0 (Wang et al 2005).
While the lung exam is important for assessing other pathologies, listening to the lungs is not an effective way to evaluate ADHF. Crackles alone are not sensitive or specific for ADHF (Wang et al 2005). [#1 thing that irritates Dr. Kittleson: basing a volume assessment on a lung exam]
Dr. Kittleson urges providers to respect sinus tachycardia, because it can indicate that there is a severe underlying condition.
Lastly, bedside ultrasound is an emerging way to augment a ADHF physical exam (Marini et al 2020).
Serially checking brain natriuretic protein (BNP) is not useful in managing ADHF (Desai 2013). BNP can be negative in HFpEF or obesity, if it’s high it could be due to renal failure or chronic ventricular dysfunction. [#2 thing that irritates Dr. Kittleson: getting called to the ER for a high BNP without a history or physical exam to accompany it.]
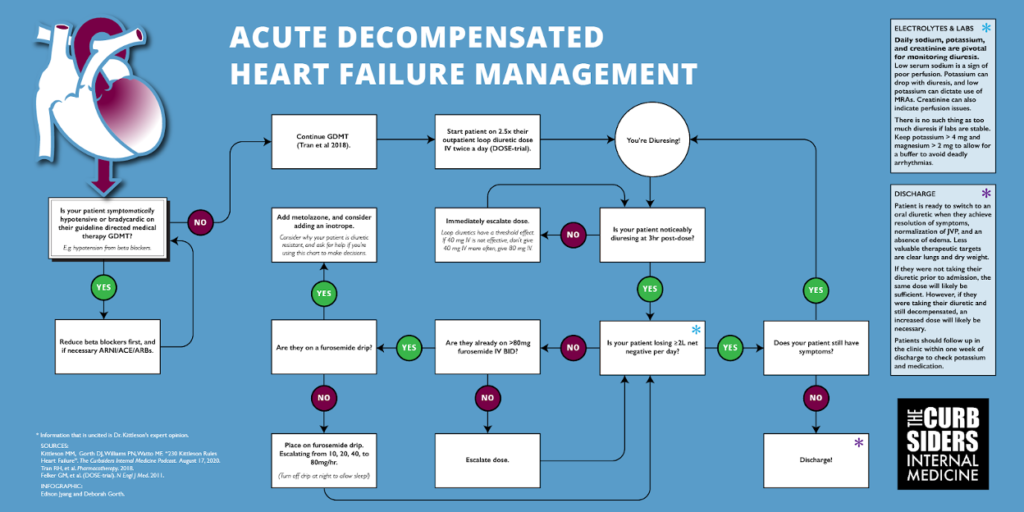
Daily sodium, potassium, and creatinine are pivotal for monitoring diuresis. Low serum sodium is a sign of poor perfusion. Potassium can drop with diuresis, and low potassium can dictate use of MRAs. Creatinine can also indicate perfusion issues.
Continue guideline directed medical therapy (GDMT) during hospitalization (Tran et al 2018). Stopping a beta-blocker may lead to worse outcomes (Prins et al 2015). However, if the patient has symptomatic hypotension or severe bradycardia, adjust anti-hypertensive medication. Additionally, if there was a recent increase in a beta blocker that could have precipitated a decompensation, reduce the dose. Prioritize medications, consider each component of GDMT as “spending blood pressure.” ARNI/ACE/ARB have an acute benefit of afterload reduction and should be prioritized over beta blockers, unless there is a compelling reason for the beta blocker like arrhythmias.
The standard of care for diuresis are loop diuretics, which block the Na+-K+-2CL– symporter in the proximal tubule leading to enhanced excretion of water along with these electrolytes. Start dosing with 2.5x outpatient dose IV twice a day (DOSE-Trial). Diurese the patient as quickly as possible, because every day that they spend in the hospital is a chance to get C. Diff. Be nice and consider the timing of diuresis to allow patients to sleep.
Check the efficacy of dose after the first few hours instead of waiting until the end of the day. If a diuretic is working, the patient will be making an obviously large volume of urine. Loop diuretics have a threshold effect. For example, if 40 mg IV is not effective, don’t give 40 mg IV more often, give 80 mg IV. Aim for a minimum target of 2 liters net negative each day, but there is no such thing as too much diuresis if labs are stable. Consider how much water a patient needs to lose, and set daily goals based on this target. Replete electrolytes as needed; keep the potassium > 4 mg and magnesium > 2 mg to allow for buffer to avoid deadly arrhythmias. Dr. Kittleson suggests that magnesium may be a wonderdrug to fix muscle aches from “furosemide-ennui”, and oral magnesium oxide is a low risk intervention.
If 80 mg IV BID is not effective at achieving diuresis goals, consider adding a furosemide drip (escalating from 10, 20, 40, to 80 mg/hr). However, turn the drip off at night (11pm to 5 am, say) to allow the patient to sleep, unless you are a sadist or your patient has a foley. If an 80 mg/hr furosemide drip is not effective, worry and add on metolazone–a thiazide diuretic that inhibits a sodium-chloride symporter in the distal convoluted tubule–for sequential blockade. It is important to figure out why this patient is so diuretic resistant, do they have severe underlying cardiac or kidney issues contributing. If creatinine is less than double baseline, a high creatinine could be a result of venous back pressure due to congestion, and it may resolve with effective diuresis.
You can start (a touch of an) inotrope empirically if creatine is moving in the wrong direction to see if that support augments diuresis. However, a right heart Swan-Ganz catheter allows you to measure filling pressures, pulmonary artery pressure, & cardiac output. If you have volume overload and renal dysfunction or progresssive hypotension, pulmonary artery catheter can help determine your course of action. Right heart catheters are a diagnostic not a therapeutic intervention. Use diuresis & inotropes to achieve the goals of RA <10 mmHg, wedge <20 mmHg, & cardiac index >2.
The patient is ready to switch to an oral diuretic when they achieve resolution of symptoms, normalization of JVP, and an absence of edema. Less valuable therapeutic targets are clear lungs & dry weight [#3 way to irritate Dr. Kittleson is saying that the pt is diuresed to dry weight and is ready to go home].
Decide on a new outpatient dose by looking at their old outpatient diuretic dose. If they were not taking their diuretic prior to admission, the same dose will probably be sufficient. However, if they were taking their diuretic and still decompensated, an increased dose will likely be necessary. Try the home oral diuretic dose for 24 hours to gain insight into how to adjust discharge dosing. Dr. Kittleson advises that a dose resulting in 500 cc negative in hospital will likely keep them net even at home. Include the admission & discharge weight in the discharge note. While self-reported dry weights are not typically accurate, admission & discharge weights will be useful during future hospitalizations. Patients should follow up in the clinic within 1 week of discharge to check potassium & medication.
Use hospitalizations as an opportunity to add on medications that are included in GDMT. SGLT-2 inhibitors are newly approved for the treatment of HF in patients even in the absence of diabetes (DAPA-HF).
When to Use a Swan-Ganz Catheter
Consider the Swan when: fluid overloaded, hypotensive, with rising Creatinine.
Swan-Ganz catheter allows you to measure filling pressures, pulmonary artery pressure, & cardiac output.
Use diuresis & inotropes to achieve the goals of RA pressure <10 mmHg, pulmonary capillary wedge pressure <20 mmHg, & cardiac index >2.
The greatest reduction in cardiovascular (CV) mortality in those with HFrEF was found with the combination of an angiotensin receptor–neprilysin inhibitor (ARNi), sodium glucose cotransporter-2 inhibitor (SGLT2i), beta blocker (BB), & a mineralocorticoid receptor antagonist (MRA). This combination was calculated as adding 7.9 years of life after age 50 & 5 years of life after age 70. The combinations with the greatest benefit included the new ARNi combination drug.
How neprilysin inhibition improves heart failure
When the heart fails, the ventricles dilate & the heart muscle stretches, stimulating the release of B-type, or brain natriuretic, peptide (BNP). This is the same peptide we measure to monitor the severity of CHF. There are 3 types of natriuretic peptide, the BNP (brain), A-type (atria), and C-type (think “circulatory”) that lines the endothelium. These peptides (BNP, ANP, CNP) act as diuretics to help reduce the harmful effects of the stretching heart muscle, while also reducing sympathetic tone. The BNP connects the heart to the brain through the sympathetic nervous system.
Neprilysin is an enzyme that lyses these natriuretic peptides, making them inactive. Inhibiting neprilysin results in more natural diuretic peptides to help the body self-treat heart failure. The challenge in giving a neprilysin inhibitor alone is that it also increases angiotensin II levels, which results in a reduction of its therapeutic effects. Adding an angiotensin receptor blocker (ARB) enhances the natriuretic effects of BNP, ANP, and CNP; this includes diuresis, sodium excretion, vasodilation, scarring prevention, & sympathetic stress reduction on the cardiovascular system.
A key pearl to remember is that once a neprilysin inhibitor is started, BNP will be falsely elevated & can no longer be used as a dependable marker for monitoring CHF.
Neprilysin inhibitors can worsen kidney failure; Therefore, reduced dosing is needed in pts with kidney failure, & potassium should be monitored due to the risk of hyperkalemia. Don’t give this drug with an ACE inhibitor as it can increase the risk of angioedema.
Summary
We have two relatively new classes of drugs, ARNi and SGLT2i, that will be playing a significant role in the treatment of HFrEF now and into the future; however, these drugs are expensive.
Cost:
I would love to see a study comparing the $18 dollar a month combination of an ACEi, BB, and MRA along with a low-salt Mediterranean diet, regular exercise, and meditation to the $1042 dollar a month combination of an ARNi, SGLT2i, BB, and MRA. However, which pharmaceutical company would pay for that?
Source: Tromp J, et al. A Systematic Review and Network Meta-Analysis of Pharmacological Treatment of Heart Failure With Reduced Ejection Fraction. JACC: Heart Failure. Volume 10, Issue 2, February 2022, Pages 73-84