
Today will feature a guest post with Leo Stemp exploring some bleeding-edge concepts. Very soon, an Internet Book of Critical Care chapter will round things out with a broader look at this disease.

Leo Stemp trained first in critical care (including medical school at Harvard, internal medicine residency at Mount Sinai, and a fellowship at Maryland Shock Trauma). But that wasn't enough for him, so he went back and completed an entirely additional training in anesthesiology at Stanford (with subsequent fellowships in both adult and pediatric cardiac anesthesia). His career path subsequently combined anesthesiology and critical care within academic and non-academic venues over more than twenty years of practice, giving him a truly unique perspective on the critically ill patient.
Below are Dr. Stemp's thoughts on the treatment of patients with asthma. I couldn't resist intermittently interjecting some thoughts of my own (in italics). If you have additional comments, please add them in the comments section below. There are a lot of important concepts flying around here – we will try to bring them all home in the upcoming IBCC chapter.
In asthmatics and COPD patients in respiratory distress, the major priority to keep in mind is that:
THE RESPIRATORY RATE MUST BE SLOWED TO A RANGE OF 15-20.
No matter what you do, the patient will not improve significantly until that is accomplished. See below for the explanation why.
The only way to accomplish that quickly is through the use of narcotics. (Fentanyl is best because it's so fast acting, but anything will do.) Once you do that, bronchodilators with or without CPAP will have a chance to work.
End of story.
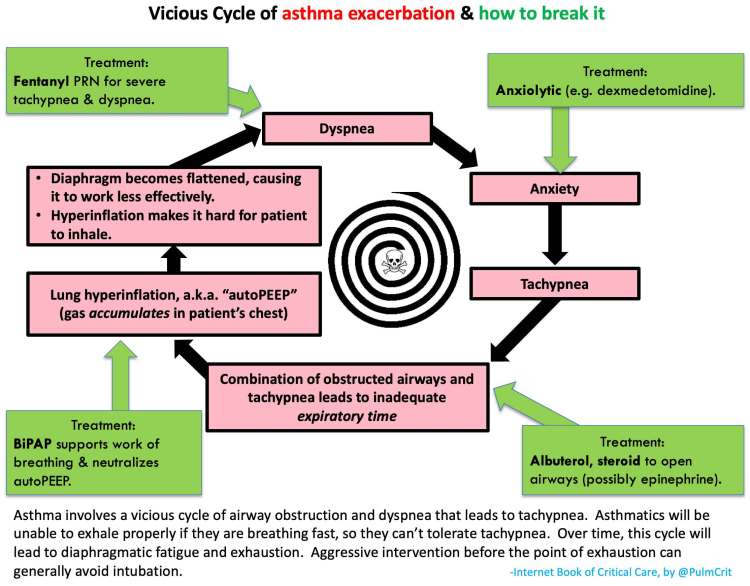
The reason that a slow respiratory rate is so crucial in asthmatics is because the problem in asthmatics & COPD patients is obstruction to exhalation. So it takes them a looooooong time to exhale. A fast respiratory rate shortens the time available for exhalation, leading to breath stacking and ultimately hyperinflation, which worsens the mechanical advantage of the inspiratory and expiratory muscles and compounds the patient's respiratory distress, resulting in a vicious cycle.
That’s why opiates should be employed early, before the opportunity is lost (see further below). A slow respiratory rate lengthens the time available for exhalation. Once the patient has enough time for exhalation, their hyperinflation will slowly deflate, so their respiratory muscles will function much better and they'll breathe much easier. In addition, a slow respiratory rate is associated with higher tidal volumes, which lowers the dead space fraction, leading to greater elimination of CO2 and reduced work of breathing.
Therefore, when an asthmatic or COPD patient comes in with tachypnea and respiratory distress, the patient should immediately be given fentanyl or another opiate to slow down their respiratory rate, in addition to the usual bronchodilators, with or without CPAP. Getting the respiratory rate down is the #1 priority. Once you do that, the patient's problems are over, period.
The use of opiates is mother's milk to these patients. It's truly magic. Treating respiratory distress in asthmatics and COPD patients is just that simple.
One further note: You have to give enough opiate to work. And sometimes, it takes a lot more than you think, because these patients are really revved up, because they’re in respiratory distress! So if you've given what you think should be enough opiate, and the respiratory rate hasn't slowed yet, you need to give more!
How do you stay out of trouble when you're giving a lot of narcotic? Simple: Keep the patient on room air, and watch the saturation! When the typical asthmatic is breathing room air, the saturation directly reflects alveolar ventilation There is no reason to do blood gasses. If the patient is hypoxemic on room air, then use as little oxygen as possible to keep the Sat close to 90% (the physiology of this is elucidated below). And you MUST watch the patient CLOSELY. This is not the kind of thing where you can give a dose of narcotic, leave the room, and check in on them every ten minutes.
A second way of staying out of trouble using opiates to treat patients in respiratory distress is to backstop them with CPAP or BiPAP. The CPAP/BiPAP machine has alarms and a set back-up respiratory rate in case the patient’s respiratory rate goes too low or the patient becomes apneic. Note, however, that in 30 years of giving opiates to patients in respiratory distress, I have yet to cause apnea or bradypnea such that back up ventilation is required. That’s not because of any special expertise, it’s because of the inherent safety of the physiology.
Some comments from Josh:
1. Use of CPAP and BiPAP in asthmatics and acute ‘tight' COPD patients with hyperinflation: As noted above, these patients have a problem with exhalation, because of narrowing of the airways (caused by bronchospasm, increased sputum production w mucus plugging, bronchial wall edema, and airway closure). Continuous positive airway pressure (CPAP) is a specific antidote for this, because it stents the airways open, promoting expiratory airflow.
Any tight asthmatic or COPD patient who is in respiratory distress, who does not respond within minutes to a bronchodilator and opiate, should be put on CPAP immediately! CPAP is the antidote. Do not wait very long to try CPAP. You want to treat these patients aggressively early, and get as far ahead of the situation as quickly as possible, because if you wait, you may lose the opportunity.
Why is the opportunity lost? Because what causes an asthmatic’s clinical condition to worsen is further narrowing of their airways, leading to worsening hyperinflation. Ultimately, their hyperinflation gets so bad, compounded by the worsening mechanical advantage of the respiratory muscles, that enough volume of air just can’t get in. That is catastrophic because the problem persists even after intubation. So the patient must be rescued before that happens! And it’s much easier to manage them if opiates and CPAP are used early, before they even get to the point of requiring rescue!
What about BiPAP? The purpose of BiPAP is to augment tidal volume over what a patient would get on ordinary CPAP. However, asthmatics do not have a problem with inhalation. Moreover, if they take too large a tidal volume, that is going to worsen their hyperinflation and make breathing more difficult!
So when it comes to CPAP vs BiPAP for these patients, always start them off with CPAP (start with a higher level of CPAP, such as 10cm, rather than just 5cm). Do not start off with BiPAP, because that could make them worse, by giving them large tidal volumes. Once you put them on CPAP and watch them for a few breaths, if their tidal volumes are too low, the patient can be switched over to BiPAP by adding 2cm of iPAP (inspiratory positive airway pressure) at a time.
The use of CPAP in these patients is magic. In most of these cases, the use of BiPAP is a myth.
[Pure asthmatics virtually never need BiPAP. The same is true for the hyperinflated COPD patient with acute bronchospasm and/or airway closure. The situation is very different though for chronic COPD patients with an exacerbation. Many of the latter will, in fact, need BiPAP. Figuring out which one is which is easy, since you can always start with CPAP and then after a few breaths, just switch over to BiPAP by adding a few cm of iPAP at a time, until the patient has a good chest rise and tidal volume. The respiratory therapists are very competent at doing this and if there's any question, my suggestion is to let them determine what the patient needs.]
Some comments from Josh:
2. NEVER, EVER, give a patient with respiratory distress a sedative, such as a benzodiazepine. If the patient looks anxious to you, they have good reason to be: They're in respiratory distress! Once you relieve their respiratory distress, their anxiety will go away. The treatment for that is fentanyl or another opiate. It's that simple.
In contrast, a sedative (such as Ativan) would only dull their mental status, which would reduce their drive to breathe, reduce their margin of safety, and reduce the margin of safety available for giving opiates.
The use of opiates in respiratory distress is magic. The use of Ativan or midazolam is a myth. (Not just a myth; it's hugely dangerous.)
Note: If despite the aforementioned, you find yourself in a situation where it strikes you that this patient is different, and Ativan is the right drug in this particular situation, just keep in mind that there's a > 99.99% chance that you're wrong. (If I gave the world $10,000 for each time Ativan was the right drug for a patient in respiratory distress*, and collected only a penny for every patient for whom an opiate was the right drug, I would be a millionaire in less than a year.)
[* By respiratory distress, I mean patients with respiratory distress due to an organic problem like asthma or COPD or CHF. I'm not talking about a patient in respiratory distress due to an anxiety attack, the latter for whom my treatment of choice would probably be a simple paper bag. Also, there are rare patients who suffer such anxiety that it produces acute severe bronchospasm and inability to move air. Our experience is that for such patients, fentanyl and CPAP will relieve their acute symptoms, and then a baseline of anti-anxiety medication (Ativan, Xanax, Klonopin, etc.) will hopefully prevent recurrence.]
Some comments from Josh:
3. Blood gasses are very rarely needed in asthmatic patients, bec the management of asthma is all clinical. The clinical picture is what dictates the clinician’s response, as outlined above, not the blood gas. There is virtually no asthmatic who needs a blood gas, until they’ve failed treatment w bronchodilators and opiates (first line), and NIV and steroids (second line), and they’re getting worse.
Accordingly, it is probably reasonable to say that a true need for a blood gas in any asthmatic should trigger a call to the ICU. (That may seem contrary to current practice, but that is only because we get way more blood gasses than needed in our asthmatics, often before both opioids and NIV have been employed.) Once the patient is in the ICU, third tier therapies can be employed, e.g. epinephrine drips, ketamine, and Heliox, in addition to much closer respiratory monitoring.
Some comments from Josh:
4. The use of supplemental oxygen in asthmatics puts the patient’s life in grave jeopardy!
That is because supplemental oxygen masks the hypoxemia that would otherwise be picked up by the SpO2 when an asthmatic patient gets so bad that they develop gross hypoventilation, resulting in hypoxemia if they’re breathing room air.
As the asthmatic patient hyper-inflates, their tidal volume becomes less and less, which means that dead space fraction increases, and alveolar ventilation drops off, resulting in a rise in the pCO2. So the bottom line pathophysiology of asthma is simple: hypoventilation. In turn, the hypoventilation causes hypoxemia. Assuming that the patient doesn’t have pneumonia, hypoxemia in asthmatics is caused by simple hypoventilation (*see a further note on hypoxemia in asthmatics, on the bottom of page 6 below).
From a practical standpoint though, there’s something unique about the hypoventilation and hypoxemia in asthmatics: In asthmatics, clinical deterioration, with worsening hypoventilation, can occur with shocking rapidity, and has a way of deteriorating unexpectedly and RAPIDLY to respiratory arrest. (That’s why asthmatics can — and do — die fast and unexpectedly at home.) Even in the hospital, the condition of a patient with severe asthma frequently deteriorates over a time span of seconds.
But it’s even worse than that, because even if intubated, the asthmatic might not be able to be ventilated! (which happened to us recently: An asthmatic was intubated, and initially we could not achieve tidal volumes much better than 180 cc — ie. just above dead space. So for hours, his pCO2 was above 150mm, and his pH was below 6.8, which are the limits of detection of the blood gas machine).
Therefore, from a practical management standpoint, in order to stay out of trouble and know when to be aggressive early, the clinician must know, at any instant, what the quantitative state of the asthmatic patient's alveolar ventilation is. Given that with today’s technology, there is no available device to continuously monitor pCO2, how then is it possible to quantitatively monitor alveolar ventilation, without, say, doing blood gases every ten minutes? Simple: By monitoring the SpO2 on room air. Here’s why:
The alveolar gas equation indicates that, for all intents and purposes, at any given FiO2, the sum of arterial pO2 and arterial pCO2 is a constant. So in an asthmatic, at any constant FiO2, as pCO2 rises because of hypoventilation, pO2 must decrease. This physiologic reality is a huge boon to clinicians because, while we don’t currently have a practical way of continuously monitoring pCO2, we do have a way of monitoring pO2 — via pulse oximetry. And if we know what the pO2 is, we therefore know, with relative quantitative certainty, what the pCO2 is, what direction the pCO2 is headed in, and how fast.
BUT: That requires that the decrease in arterial pO2 caused by hypoventilation be reflected by the pulse oximeter reading (the SpO2). That in turn is only the case if the pO2 is in the downsloping area of the Hb-oxygen dissociation curve. The diagram below shows that that area on the Hb-oxygen dissociation curve occurs when the pO2 is less than roughly 80-90mm or so:
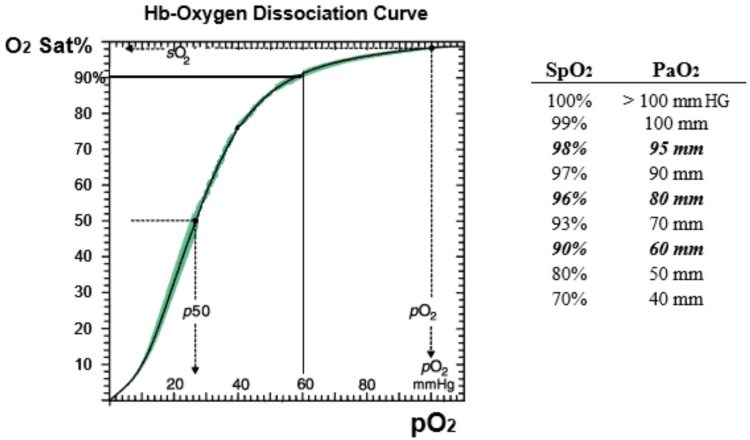
Most asthmatics have basically healthy lung parenchyma, ie. they don’t have hypoxemic lung disease (like pneumonia or CHF). Therefore, on room air, at baseline, they’ll have a pO2 in the range of, say, 80-95, which translates to a SpO2 of about 96-98%.
So in an asthmatic, once the pCO2 starts to rise, the pO2 will fall. If they’re on room air, that will be directly and immediately reflected in a drop in the SpO2. E.g., if the pCO2 rises by 10mm, that would cause the pO2 to drop about 10mm, from, say, 80mm to 70mm, which would cause the Sat to drop from 96% to 93%. And the further the pCO2 falls, the faster and further the SpO2 will fall. In that case, the clinician knows, in a semi-quantitative fashion, that the pCO2 is rising, and roughly by how much. That in turn tells the clinician how bad the patient’s asthma is.
The key to remember is that the use of the SpO2 to make inference about the pCO2 and alveolar ventilation is predicated on a starting pO2 in the 90mm range or less. That range would occur in either a patient breathing room air, or a patient breathing oxygen who has a Sat in the mid 90’s or less to start. In contrast, if the patient is breathing supplemental oxygen and has a pO2 in the mid hundreds, then in that case, even if hypoventilation drops their pO2 by 50 mm, it’s not going to be reflected in a drop in the SpO2! (the SpO2 would probably stay in the range of 99-100%).
Bottom line: All patients with asthma should be monitored with pulse oximetry while breathing either room air, or a low enough FiO2 to keep their Sat no higher than the mid 90’s. The requirement for oxygen in a (pure) asthmatic needs to scare the s*#! out of you, because asthmatics can and do die suddenly. Therefore, an acute asthmatic who is hypoxemic on room air requires CLOSE MONITORING, at least until their period of danger is deemed clinically to have passed. Should an acute asthmatic be hypoxemic on NIV, then the patient needs to be transferred to ICU immediately.
(Note: I think anybody can tell when an asthmatic is in respiratory distress. But who are the acute asthmatics who bear close monitoring in the intermediate care unit (step down ICU), as opposed to the regular medical ward? One clue might be the requirement for oxygen, although that, in itself, doesn't tell the whole story. That is the subject of clinical judgment, to be discussed with the patient's physician. Regardless, a requirement for oxygen in a pure asthmatic indicates a worse condition, and therefore requires closer monitoring. That's a simple, unequivocal fact of nature.)
A quick digression into history here for the sake of completeness: It has long been known that, based on blood gas patterns, the progression of acute asthma can be grouped into four stages:
Stage 1 – pCO2 is low, pO2 is normal-to-high: In early asthma, the patient can still move air well. Reflex receptors in the lung, together with CNS excitation, trigger hyperventilation, which lowers the pCO2. In turn, pO2 is normal (or even high, because of the alveolar gas equation — see below). The hallmark of this stage of asthma is the low pCO2. If you do an ABG on an asthmatic, this is the kind of blood gas you want to see.
Stage 2 – pCO2 is normal, pO2 is normal: As asthma progresses, tidal volume starts to drop off (as explained earlier and also below). But the increase in respiratory rate is able to maintain a normal pCO2. According to the alveolar gas equation, pO2 is expected to be normal. The hallmark of this stage of asthma is the normal pCO2. BEWARE OF THIS BLOOD GAS PATTERN! A stage 2 asthmatic has an entirely normal looking blood gas — which tends to lull the clinician into thinking everything is OK. But it’s not OK! A normal pCO2 in an asthmatic needs to alarm the clinician because it means that the patient is well into stage 2. This is NOT the kind of blood gas you want to see in an asthmatic.
People who do a lot of ABGs in asthmatics see mostly stage 1 and stage 2.
Stage 3 – pCO2 is high, pO2 is low-normal: As asthma progresses further, gross hypoventilation ensues and for the first time, the pCO2 rises into the above-normal range. Even a mildly highish pCO2 in an asthmatic has a very, very different ramification than a similar pCO2 in the usual respiratory failure patient, for the reasons noted in section 4 above — specifically, because of how fast the asthmatic can deteriorate, and the calamity that it is when they have to be intubated and still can’t be ventilated. The hallmark of this stage of asthma is the high pCO2. Asthmatics with a high pCO2 need urgent attention and escalation of treatment, and probably transfer to the ICU if their clinical condition doesn’t improve in short order.
Stage 4 – pCO2 is high, pO2 is frankly low: As asthma progresses further, the pCO2 rises to such a degree that, for the first time, as dictated by the alveolar gas equation, the pO2 and therefore the SpO2 are frankly hypoxemic (on room air. Supplemental oxygen would obviously raise the pO2). The hallmark of this stage of asthma is the hypoxemia*. This classic pattern occurs right before the asthmatic is about to die (see further discussion of hypoxemia in asthma, below).
This pattern of blood gasses, and the stages of asthma, were described in the early days of blood gas measurement, in the mid-1900’s. At that time, the management of asthma and respiratory failure was primitive, and many patients with asthma did in fact die.
[* In reality, a lower-than-normal SpO2 on room air is common in even mild-moderate acute asthma, and clearly those patients don’t have stage 4 asthma. In fact, their mild desaturation is not entirely because of hypoventilation. Rather, shunting caused by small airway closure is the cause of the lowered pO2. Small airway closure in asthmatics is caused by a combination of bronchoconstriction, bronchial edema, and mucus plugging, and results in perfusion without ventilation, ie. V/Q mismatch and shunting. So a MILDLY decreased room air Sat in asthmatics is common, and is due to a combination of hypoventilation and V/Q mismatch. But it should not go further than that. In an asthmatic who otherwise has no parenchymal lung disease (e.g. no pneumonia or emphysema), a drop in their room air Sat into the low 90’s or below represents a dangerous situation, and should be managed aggressively.]
Some comments from Josh:
5. VENTILATOR MANAGEMENT of the intubated acute asthmatic/COPD patient:
In the very unfortunate situation where the acute asthmatic type of patient winds up intubated, the same principle holds true: The respiratory rate must be kept slow to give the patient a longer time interval for exhalation. Accordingly, the best mode of ventilation for these patients is CPAP with Pressure Support, along with enough fentanyl to keep their resp rate in the 15 range (less than 20). This allows the patient to regulate their own expiratory phase, which is much better than trying to manage their expiratory phase by guessing at the best ventilator settings. (IMO, these patients should always be managed with pressure-cycled ventilation, not volume cycled. Again, I'm talking about the acute, tight/wheezy patient, not the chronic COPD patient.)
In the even more unfortunate event that such a patient needs to be paralyzed to achieve adequate ventilation, the resp rate, delta, and especially the I:E ratio must be juggled to achieve the best alveolar minute ventilation at the lowest plateau pressure possible (with the PEEP setting being determined by matching the auto‑peep.) End-tidal CO2 monitoring should be deployed in these patients as soon as possible after intubation, and a blood gas should be obtained within one hour to determine the end-tidal CO2 vs blood gas pCO2 difference. After you have that information, the end-tidal CO2 can be used to guide subsequent ventilator settings to achieve the best ventilation and pCO2.
Some comments from Josh:
6. Use of KETAMINE in the management of acute asthma:
It is not the purpose of these notes to be an exhaustive treatise on the management of acute asthma. But as the use of ketamine for acute asthma in the ED seems to be in fashion nowadays, I offer the following comments:
First line ED management of acute asthma is with bronchodilators and steroids. Why? Because they’re simple and easy, they work, they’re predictable, and they’re uncomplicated.
Second line ED management of acute asthma is with opiates and CPAP. Why? Because they’re simple and easy, they work, they’re very predictable, and they’re uncomplicated.
All other therapies after that have to be considered third line and beyond, because they’re not simple and they’re not uncomplicated. That includes epinephrine and ketamine (in that order).
In specific regard to ketamine: Ketamine has sedative effects that would be undesirable (as noted above), and worse, dysphoric effects (even in low dose) that can be extraordinarily troubling to patients (speaking from large experience, as an anesthesiologist). Thus, while ketamine may have bronchodilatory properties that may or may not have some salutary role in the rescue of acute asthmatic patients, decades of unequivocal clinical success with opiates and CPAP put ketamine a long way down the line in the armamentarium available to the ED physician to treat asthmatics with respiratory failure.
A 2011 article in the Journal of Emergency Medicine warrants further comment: Shlamovitz GZ, Hawthorne T: Intravenous ketamine in a dissociating dose as a temporizing measure to avoid mechanical ventilation in adult patient with severe asthma exacerbation. J Emerg Med 2011;41:492–4.
The authors’ ‘dissociative’ dose of ketamine, 0.75 mg/kg (and I’m assuming that’s ideal body weight, not actual body weight), is most definitely an anesthetic dose in a patient with acute respiratory failure. I’m a bit alarmed by that idea, as being both a critical care physician and an anesthesiologist, the last thing I would do to a patient who is hypoxemic with the kind of respiratory failure they described would be to administer an anesthetic dose, of any agent (unless it was immediately prior to tracheal intubation). That’s a paradigm that would be highly unusual in the entire realm of clinical medicine. Not something to be undertaken lightly. And even though ketamine is known to maintain spontaneous ventilation and hemodynamics, the effect of an anesthetic dose of ketamine on a patient in extremis is entirely unpredictable (speaking from large experience). On the other hand, if the authors administered ketamine as a last ditch effort to avoid intubation while they stood over her with laryngoscope in hand, that, in fact, might be an excellent idea (the ketamine serving as the induction dose for intubation, if it failed to work to salvage the patient).
Although the patient described in the authors’ case report had an excellent outcome, ketamine has failed to consistently produce such results, as the authors’ own literature search revealed. Furthermore, it cannot even be concluded that bronchodilation was the reason for ketamine’s success in their case. Other conceivable mechanisms of actions include a relaxation of chest musculature, or slowing of the respiratory rate, either one secondary to ketamine’s sedative/anesthetic effect.
Bottom line, the efficacy and side effect profile of ketamine doesn’t compare to the predictable efficacy and safety of opiates and CPAP. Ketamine should be reserved for patients who fail standard therapies. It’s use in anesthetic doses as rescue therapy in acute asthma with respiratory failure should be undertaken only with immediate readiness to address both respiratory and circulatory collapse.
Some comments from Josh:
That was a long post, so congrats to anyone who made it this far. I'm looking forward to the discussion around these ideas here, and in the upcoming IBCC chapter. Asthma is a nexus of many things which resuscitationists cannot agree on, which makes it interesting.
I enjoyed this discussion & just have to say you’re both right although it’s not that simple.! Scott, your opinion on the “semi-crashing” asthmatic is only somewhat correct. These type of patients are often exhausted b/c they’ve “trapped” some air in their chest & with that comes increased work of breathing, if it progresses they get to the point where they can’t do it anymore. Try this yourself, take 1/2 vital capacity breath, hold don’t exhale, then continue to vital capacity. Follow this with 3/4 vital capacity breath, hold, then continue to vital capacity. Repeat this sequence 5-10 times & see if how you feel about keeping it going. You’ll appreciate the work of breathing is significant. If you don’t exhale enough, b/c with higher respiratory rate, you will tucker out. As soon as CO2 gets out of range, sympathetic drive increases. The “semi-crashing” asthmatic has to slow down & get a bit of help to overcome their increased work of breathing. They are like you said technically having difficulty inspiring, however they also have “breath stacked” significant auto-peep and can’t expire effectively either. If this vicious cycle continues, they will be “crashing asthmatics”. Respiratory mechanics is very complex, & we have various shapes/sizes as well, with no two patients the same. I watch ventilator flow volume loops everyday at work, & vents have to be “tweeked” for optimal +ve pressure breathing. Fortunately with better therapy these days, we rarely see the hypoxemic, hypercarbic, near dead asthmatics anymore. These folks can’t tidal breath in or out & over dead space ventilating ends badly.
I think ketamine is the best drug for “semi-crashing” patients as well, however use caution in the patient with concurrent URTI/chest infection. Ketamine is a 4 star drug, however increased dosing will cause hypersalivation which will lead to problems. Ketamine as profound opioid sparing effects, though it’s not an opioid, & adding “bit” of fentanyl to decrease sympathetic tone is effective.