
Atelectasis and Overdistension video
Go to: Fick's Law for Diffusion
Hysteresis (the difference between the inflation curve and the deflation curve) indicates energy loss.
Uptake of oxygen and excretion of carbon dioxide require rapid, efficient exchange in the lung. The quantities of exchanged gases are staggering. For example, a 1800-calorie diet requires absorption of 375 L of oxygen per day, as well as excretion of a slightly smaller volume of carbon dioxide. Because blood remains in the pulmonary capillary bed for a limited time, the process of exchange must be accomplished in < 0.75 second at rest and 0.5 second during exercise. This rapid, high-volume exchange occurs efficiently despite numerous interacting processes of diffusion and chemical reaction that occur in the lung. The rates of these processes are not only affected by intrinsic characteristics of blood but also determined by a host of other factors, including inspired oxygen fraction, alveolar gas tensions, cardiac output, & metabolic activity. The ease of exchange of respiratory gases belies the complexity of the overall process.
Define diffusion, and distinguish it from “bulk flow."
Diffusion of a gas occurs when there is a net movement of molecules from an area in which that particular gas exerts a high partial pressure to an area in which it exerts a lower partial pressure. Movement of a gas by diffusion is therefore different from the movement of gases through the conducting airways, which occurs by “bulk flow” (mass movement or convection).
During bulk flow, gas movement results from differences in total pressure, and molecules of different gases move together along the total pressure gradient.
During diffusion, different gases move according to their own individual partial pressure gradients. Gas transfer during diffusion occurs by random molecular movement. It is therefore dependent on temperature because molecular movement increases at higher temperatures. Gases move in both directions during diffusion, but the area of higher partial pressure, because of its greater number of molecules per unit volume, has proportionately more random “departures.” Thus, the net movement of gas is dependent on the partial pressure difference between the two areas. In a static situation, diffusion continues until no partial pressure differences exist for any gases in the two areas; in the lungs, oxygen and CO2 continuously enter & leave the alveoli, and so such an equilibrium does not take place.
The concentration (C) of a gas dissolved in fluid depends upon its partial pressure (P) and solubility (α): 
Gases diffuse from a higher to a lower partial pressure, not necessarily from a higher to a lower concentration. This fact is especially pertinent when a gas diffuses between two phases, as occurs when O2 and CO2 are exchanged between alveolar gas and blood. For example, dissolved CO2 diffuses down a partial pressure gradient from blood (46 mm Hg) into the alveolus (40 mm Hg), even though its actual concentration (millimoles of molecular CO2 per liter of gas or blood) is greater in alveolar gas (2.5) than it is in venous blood (1.4).
Influence of Physical Properties
The rate of a gas diffusing through an aqueous membrane such as that separating alveolar gas and capillary blood is influenced by five factors. The rate is directly proportional to the surface area of the membrane, but inversely proportional to the thickness of the membrane. The rate increases in direct proportion to the difference in gas pressure between alveolar gas and capillary blood, and the diffusion and solubility coefficients of the gas in the membrane.
The diffusion coefficient of a gas in the alveolar–capillary membrane is largely a function of the size of the gas molecule, which is inversely proportional to the square root of its molecular weight (MW). Oxygen (MW 32) has a slightly greater diffusion coefficient than carbon dioxide (MW 44) in the alveolar membrane. However, the solubility of CO2 in water, the major component of tissue composing the membrane, is much greater than the solubility of O2. This difference far outweighs the effect of the slightly smaller size of the oxygen molecule. Thus, the rate of CO2 transfer across the alveolar membrane is approximately 20 times greater than that of O2 when both gases diffuse under the same partial pressure gradient. As a result, a much greater PO2 gradient across the membrane is required to maintain O2 transfer equal to that of CO2.
On the other hand, the rate of carbon monoxide (CO) transfer is very similar to that of oxygen when both gases diffuse across the alveolar–capillary membrane under the same partial pressure gradient. CO (MW 28) is a slightly smaller molecule than oxygen so its diffusion coefficient is slightly greater. This diffusive advantage is offset by a slightly lower aqueous solubility of CO compared to oxygen. As a result, CO and O2 transfer across the membrane have approximately equal rates at the same transmembrane partial pressure gradient.
The rate of diffusion is affected by the viscosity of the medium through which the diffusion occurs. Diffusion of a gas in air occurs at a rate that is four orders of magnitude greater than diffusion in water. Diffusion coefficients in tissues are only moderately less than those in water, since most tissues are composed primarily of water. The interior of the erythrocyte is an exception to this general rule. As a consequence of the high concentration of hemoglobin inside the red cell, the viscosity of the cell contents is substantially greater than that of water. This greater viscosity reduces the diffusion coefficient for oxygen to one-third of its aqueous coefficient. The combination of increased viscosity & the large size of the hemoglobin molecule decreases the diffusion coefficient of hemoglobin within the red cell to < 10% of its diffusion coefficient in a dilute aqueous solution. As a result, significant diffusion gradients are thought to exist within the red cell even though the distance between the cell membrane & the innermost portion of the cell is only a few microns.
Effect of Different Capacitances
The alveolar–capillary membrane provides a barrier to diffusion of gases between the alveoli and the capillaries. The rate of approach to diffusion equilibrium of a gas in the lung is dependent on the capacitances of the gas in the alveoli & blood relative to its solubility in the alveolar–capillary membrane. Normal ventilation of alveoli results in a large reservoir of oxygen with a pressure of ∼100 mm Hg to promote diffusive transfer across the alveolar–capillary membrane. The ability of hemoglobin to bind O2 increases the oxygen capacity of blood by two orders of magnitude compared with that of the alveolar–capillary membrane. This large capacitance for oxygen in blood requires substantial oxygen transfer across the membrane to reach diffusion equilibrium. Because the solubility of oxygen in the membrane is small relative to the large capacitances in alveolar gas and blood, oxygen exchange across the membrane requires 0.2 to 0.4 second to reach equilibrium.1 Fortunately, this delay in reaching equilibrium is less than the average of 0.75 second that blood remains in the pulmonary capillary bed. These same conditions are present during carbon monoxide transfer. The large capacitance and the low aqueous solubility of CO lead to similar impediments in gas exchange.
In contrast to oxygen and carbon monoxide, the solubility of carbon dioxide in the membrane is sufficiently great compared to the capacitances of CO2 in blood & alveoli to permit rapid equilibration of CO2 across the alveolar–capillary membrane. As discussed, CO2 exchange requires a finite time for completion, but this delay is the result of the time needed to complete chemical & transport processes in blood, and is not the result of slow diffusive transport across the alveolar–capillary membrane.
Gases transported in blood only in dissolved form are exchanged almost instantaneously across the alveolar–capillary membrane. As long as gas solubilities in the membrane and blood are similar, diffusion equilibrium between alveolar contents and blood is reached within 0.01 second because the normal alveolar–capillary membrane is extremely thin (median thickness of 0.3 μm). Only gases such as oxygen & carbon monoxide that have large alveolar and blood capacitances and reduced solubility in the alveolar–capillary membrane will require a finite time to reach diffusive equilibrium.
O2 is brought into the alveoli by bulk flow through the conducting airways. When air flows through the conducting airways during inspiration, the linear velocity of the bulk flow decreases as the air approaches the alveoli. This is because the total cross-sectional area increases dramatically in the distal portions of the tracheobronchial tree, as was seen in Figure 1−2. The linear velocity of bulk flow through a tube is equal to the flow divided by the cross-sectional area:
++
Linear velocity (cm / s) = Flow (cm3 / s) ÷ Cross-sectional area (cm2 )
++
By the time the air reaches the alveoli, bulk flow probably ceases, & further gas movement occurs by diffusion. O2 then moves through the gas phase in the alveoli according to its own partial pressure gradient. The distance from the alveolar duct to the alveolar-capillary interface is usually less than 1 mm. Diffusion in the alveolar gas phase is believed to be greatly assisted by the pulsations of the heart & blood flow, which are transmitted to the alveoli & increase molecular motion.
O2 then diffuses through the alveolar-capillary interface. It must first, therefore, move from the gas phase to the liquid phase, according to Henry’s law, which states that the amount of a gas absorbed by a liquid with which it does not combine chemically is directly proportional to the partial pressure of the gas to which the liquid is exposed & the solubility of the gas in the liquid. O2 must dissolve in & diffuse through the thin layer of pulmonary surfactant, the alveolar epithelium, the interstitium, & the capillary endothelium, as was shown in Figure 1−5 (step 2, near the arrow). It must then diffuse through the plasma (step 3), where some remains dissolve & the majority enters the erythrocyte and combines with hemoglobin (step 4). The blood then carries the O2 out of the lung by bulk flow & distributes it to the other tissues of the body, as shown in Figure 1−1. At the tissues, O2 diffuses from the erythrocyte through the plasma, capillary endothelium, interstitium, tissue cell membrane, & cell interior and into the mitochondrial membrane. The process is almost entirely reversed for CO2, as shown in Figure 1−1.
++
The factors that determine the rate of diffusion of gas through the alveolar-capillary barrier are described by Fick’s law for diffusion, shown here in a simplified form:

That is, the volume of gas per unit of time moving across the alveolar-capillary barrier is directly proportional to the surface area of the barrier, the diffusivity, & the difference in concentration between the two sides, but is inversely proportional to the barrier thickness.
The surface area of the blood-gas barrier is believed to be at least 70 m2 in a healthy average-sized adult at rest. That is, about 70 m2 of the potential surface area is both ventilated and perfused at rest. If more capillaries are recruited, as in exercise, the surface area available for diffusion increases; if venous return falls, for example, because of hemorrhage, or if alveolar pressure is raised by positive-pressure ventilation, then capillaries may be derecruited and the surface area available for diffusion may decrease.
++
The thickness of the alveolar-capillary diffusion barrier is only about 0.2 to 0.5 μm. This barrier thickness can increase in interstitial fibrosis or interstitial edema, thus interfering with diffusion. Diffusion probably increases at higher lung volumes because as alveoli are stretched, the diffusion distance decreases slightly (and also because small airways subject to closure may be open at higher lung volumes).
++
The diffusivity, or diffusion constant, for a gas is directly proportional to the solubility of the gas in the diffusion barrier and is inversely proportional to the square root of the molecular weight (MW) of the gas:
++
D ∝ (solubility)/√MW
++
The relationship between solubility and diffusion through the barrier has already been discussed. The diffusivity is inversely proportional to the square root of the MW of the gas because different gases with equal numbers of molecules in equal volumes have the same molecular energy if they are at the same temperature. Therefore, light molecules travel faster, have more frequent collisions, & diffuse more rapidly. Thus, Graham’s law states that the relative rates of diffusion of two gases are inversely proportional to the square roots of their MWs, if all else is equal.++
For the two gases of greatest interest in the lung,
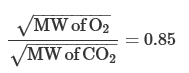
Because the relative diffusion rates in the gas phase are inversely proportional to the ratio of the square roots of their MWs,
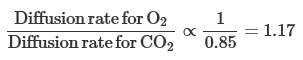
That is, because O2 is less dense than carbon dioxide, it should diffuse 1.2 times as fast as carbon dioxide (which it does as it moves through the alveoli). In the alveolar-capillary barrier, however, the relative solubilities of O2 and carbon dioxide must also be considered. The solubility of carbon dioxide in the liquid phase is about 24 times that of O2, and so carbon dioxide diffuses about 0.85 × 24, or about 20 times, more rapidly through the alveolar-capillary barrier than does O2. For this reason, patients develop problems in O2 diffusion through the alveolar-capillary barrier before carbon dioxide retention due to diffusion impairment occurs.
LIMITATIONS OF GAS TRANSFER
The factors that limit the movement of a gas through the alveolar-capillary barrier, as described by Fick’s law for diffusion, can be divided into 3 components: the diffusion coefficient, the surface area & thickness of the alveolar-capillary membrane, & the partial pressure difference across the barrier for each particular gas. The diffusion coefficient, as discussed in the previous section, is dependent on the physical properties of the gases and the alveolar-capillary membrane. The surface area and thickness of the membrane are physical properties of the barrier, but they can be altered by changes in the pulmonary capillary blood volume, the cardiac output, or the pulmonary artery pressure; by changes in lung volume; or by diseases such as fibrosis or emphysema. The partial pressure difference of a gas (across the barrier) is the final major determinant of its rate of diffusion. The partial pressure of a gas in the mixed venous blood and in the pulmonary capillaries is just as important a factor as its alveolar partial pressure in determining its rate of diffusion.
Diffusion Limitation++
An erythrocyte & its attendant plasma spend an average of about 0.75 to 1.2 seconds inside the pulmonary capillaries at resting cardiac outputs. This time can be estimated by dividing the pulmonary capillary blood volume by the pulmonary blood flow (expressed in milliliters per second). Some erythrocytes may take less time to traverse the pulmonary capillaries; others may take longer. Figure 6−1 shows schematically the calculated change with time in the partial pressures in the blood of 3 gases: oxygen, carbon monoxide, and nitrous oxide. These are shown in comparison to the alveolar partial pressures for each gas, as indicated by the dotted line. This alveolar partial pressure is different for each of the 3 gases, & it depends on its concentration in the inspired gas mixture & on how rapidly it is removed by the pulmonary capillary blood. The schematic is drawn as though all 3 gases were administered simultaneously, but this is not necessarily the case. Consider each gas as though it were acting independently of the others.
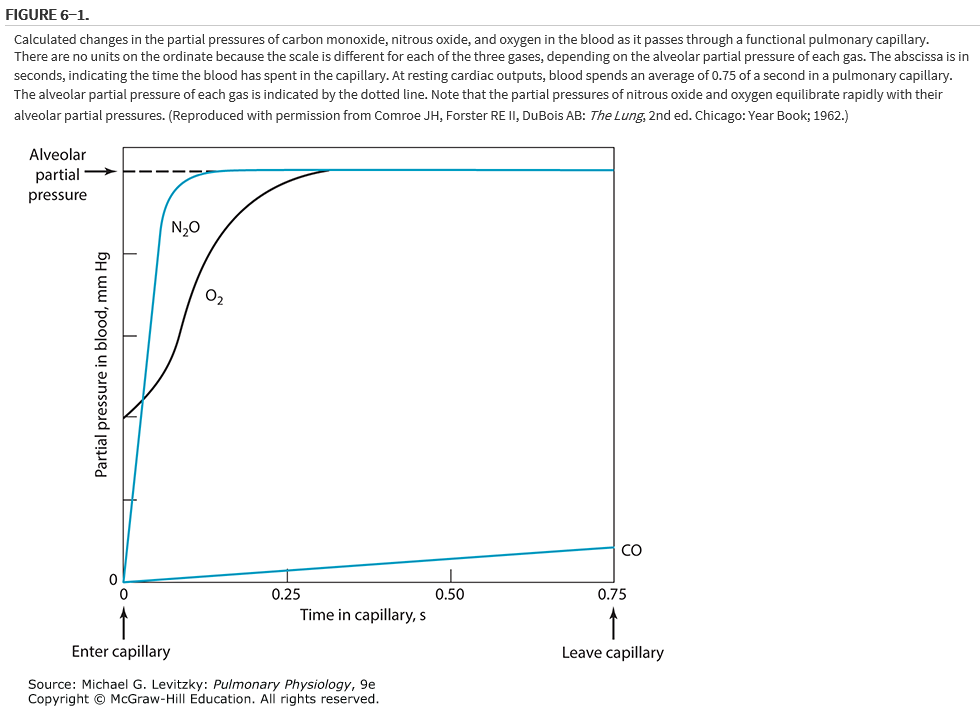
The partial pressure of carbon monoxide in the pulmonary capillary blood rises very slowly compared with those of the other two gases in the figure. (Obviously, a low inspired concentration of carbon monoxide must be used for a very short time in such an experiment.) Nevertheless, if the content of carbon monoxide (in milliliters of carbon monoxide per milliliter of blood) were measured simultaneously, it would be rising very rapidly. The reason for this rapid rise is that carbon monoxide combines chemically with the hemoglobin in the erythrocytes. Indeed, the affinity of carbon monoxide for hemoglobin is about 210 times that of oxygen for hemoglobin. The carbon monoxide that is chemically combined with hemoglobin does not contribute to the partial pressure of carbon monoxide in the blood because it is no longer physically dissolved in it. Therefore, the partial pressure of carbon monoxide in the pulmonary capillary blood does not come close to the partial pressure of carbon monoxide in the alveoli during the time that the blood is exposed to the alveolar carbon monoxide. (If the alveolar partial pressure of carbon monoxide were great enough to saturate the hemoglobin, the pulmonary capillary partial pressure would rise rapidly.) The partial pressure difference across the alveolar-capillary barrier for carbon monoxide is thus well maintained for the entire time the blood spends in the pulmonary capillary, and the diffusion of carbon monoxide is limited only by its diffusivity in the barrier and by the surface area and thickness of the barrier—that is, the diffusion characteristics of the barrier itself. Carbon monoxide transfer from the alveolus to the pulmonary capillary blood is referred to as diffusion-limited rather than perfusion-limited.
Perfusion Limitation++
The partial pressure of nitrous oxide in the pulmonary capillary blood equilibrates very rapidly with the partial pressure of nitrous oxide in the alveolus because nitrous oxide moves through the alveolar-capillary barrier very easily & because it does not combine chemically with the hemoglobin in the erythrocytes. After only about 0.1 of a second of exposure of the pulmonary capillary blood to the alveolar nitrous oxide, the partial pressure difference across the alveolar-capillary barrier has been abolished. From this point on, no further nitrous oxide transfer occurs from the alveolus to that portion of the blood in the capillary that has already equilibrated with the alveolar nitrous oxide partial pressure; during the last 0.6 to 0.7 of a second, no net diffusion occurs between the alveolus and the blood as it travels through the pulmonary capillary. Of course, blood just entering the capillary at the arterial end will not be equilibrated with the alveolar partial pressure of nitrous oxide, and so nitrous oxide can diffuse into the blood at the arterial end. The transfer of nitrous oxide is therefore perfusion-limited. Nitrous oxide transfer from a particular alveolus to one of its pulmonary capillaries can be increased by increasing the cardiac output and thus reducing the amount of time the blood stays in the pulmonary capillary after equilibration with the alveolar partial pressure of nitrous oxide has occurred. (Because increasing the cardiac output may recruit previously unperfused capillaries, the total diffusion of both carbon monoxide and nitrous oxide may increase as the surface area for diffusion increases.)
Sources:
Fishman's Pulmonary Diseases and Disorders, 5e
FRC is the neutral or equilibrium point of the respiratory system. (Kaplan)
Decreases in FRC are primarily due to decreases in the outward pull of the chest wall.
FRC is influenced by the relationship between the elastic inward recoil of the lungs and the elastic outward recoil of the chest wall.
Changes in chest wall recoil occur over time in people with tetraplegia and are due to patients’ inability to regularly expand the chest wall to large lung volumes.
During periods of acute respiratory illness reductions in FRC are common & due to underlying lung pathology, eg, asthma.
Reductions in FRC predispose patients to atelectasis. If closing capacity is higher than FRC, the alveoli in dependent regions of the lung collapse on expiration. This occurs during normal tidal breathing, trapping air & precipitating atelectasis.
Source: Respiratory management. Lisa Harvey BAppSc, GradDipAppSc(ExSpSc), MAppSc, PhD, in Management of Spinal Cord Injuries, 2008
FRC is the volume of air left in the lungs at the end of a normal expiration. It is the combination of residual volume (RV) & the expiratory reserve volume. RV is the amount of air that cannot be expelled from the lungs at the end of a forced expiration. A 70-kg man would have FRC of ~ 2.4 liters.
Causes of decreased FRC
FRC decreases when there is an alteration in the elastic recoil relationship between the lungs & the chest wall. Either there is an:
Reduced FRC can be the result of widespread volume loss, e.g. following abdominal surgery, or more localized loss, e.g lobar collapse. As FRC decreases towards residual volume a point is reached where dependent airways begin to close (closing volume) & remain closed during normal tidal breathing. Gas becomes trapped distal to the closed part of the airway & is rapidly absorbed.
Loss of FRC leads to:
At normal FRC the lungs operate on the steep part of the pressure/volume (compliance) curve – therefore small changes in distending pressure (by the inspiratory muscles) easily produce an increase in volume (Fig. 7.3A).
At low FRC, lung compliance is reduced (Fig. 7.3B). Tidal volume is less for the same amount of distending pressure. To produce the same tidal volume at low FRC greater inspiratory muscle effort is required – i.e. breathing is harder work. Collapsed lung needs large distending pressures to reinflate it.
Remember
FRC is important to preserve the surface area for:
& therefore oxygenation.
Source: Bernadette Henderson, Nell Clotworthy. The management of volume loss. Respiratory Physiotherapy (Second Edition), 2009
Emphysema: reduces lung elastic recoil pressure
Chest wall disorders: (obesity, kyphoscoliosis, fibrothorax)
Pulmonary Embolism vs Airway Obstruction
The key distinction between pulmonary embolism & airway obstruction is that in PE, there is an area of blood-flow obstruction, whereas in airway obstruction the airway is obstructed. When blood flow is obstructed in the lung as in PE, the body begins to hyperventilate. Despite the physiologic dead-space created by the PE, in most cases, overall minute ventilation still increases relative to baseline, causing increased amounts of CO2 to leave the lungs (remember CO2 is not diffusion-limited & its rate of clearance is dependent on the rate of ventilation) & causing respiratory alkalosis. Conversely, with airway obstruction, less CO2 can leave the lungs despite adequate perfusion, creating a shunt. CO2 builds up in the blood & causes an acidosis.
Dead space is the volume of a breath that does not participate in gas exchange. It is ventilation without perfusion. Physiologic or total dead space is the sum of anatomic dead space & alveolar dead space. Anatomic dead space is the volume of gas within the conducting zone (as opposed to the transitional & respiratory zones) and includes the trachea, bronchus, bronchioles, & terminal bronchioles; it is ~ 2 mL/kg in the upright position. Alveolar dead space is the volume of gas within unperfused alveoli (& thus not participating in gas exchange either); it is usually negligible in the healthy, awake patient. The ratio of physiologic dead space to tidal volume is usually ~ 1/3.
Factors that increase dead space:
Sources:
https://step1.medbullets.com/user/dashboard?id=all&specialty=117&menu=topic&expandLeftMenu=true
https://www.openanesthesia.org/aba_respiratory_function_-_dead_space/
What is your understanding of the predominant mechanism by which supplemental O₂ leads to increased pCO₂ in patients with acute exacerbations of COPD?
Summary:
In 1980, the predominance of the "loss of hypoxic drive" theory was challenged by Aubier et al. This group studied 22 patients with COPD and acute respiratory failure. Baseline values: pO₂ = 38 mmHg (SaO₂ ~72%) pCO₂ = 65 mmHg
After administering 100% FiO₂, they noted the following:
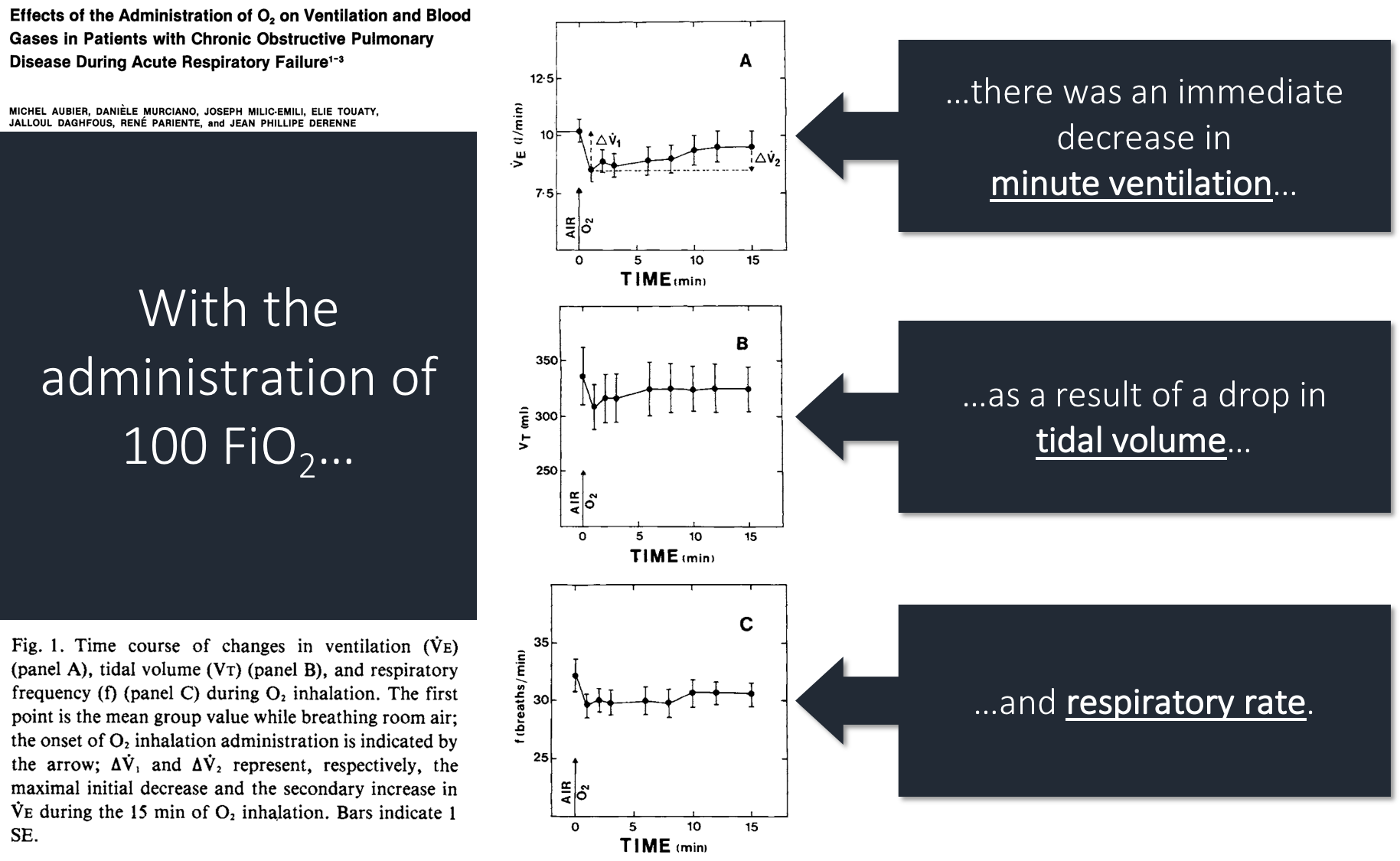
But, you'll notice that ventilation returned to 93% of baseline by 15 minutes. The authors conclude that the increase in pCO₂ can be attributed to: loss of hypoxic vasoconstriction
Why would the loss of hypoxic vasoconstriction lead to an increase in pCO₂?
With the loss of hypoxic vasoconstriction, blood flow (Q) is redirected away from areas of high ventilation (V). The fall in Q leads to: increased regional V/Q & increased deadspace.
Consistent with this, Aubier found that deadspace ventilation increased from 77% to 82% after administration of O₂.
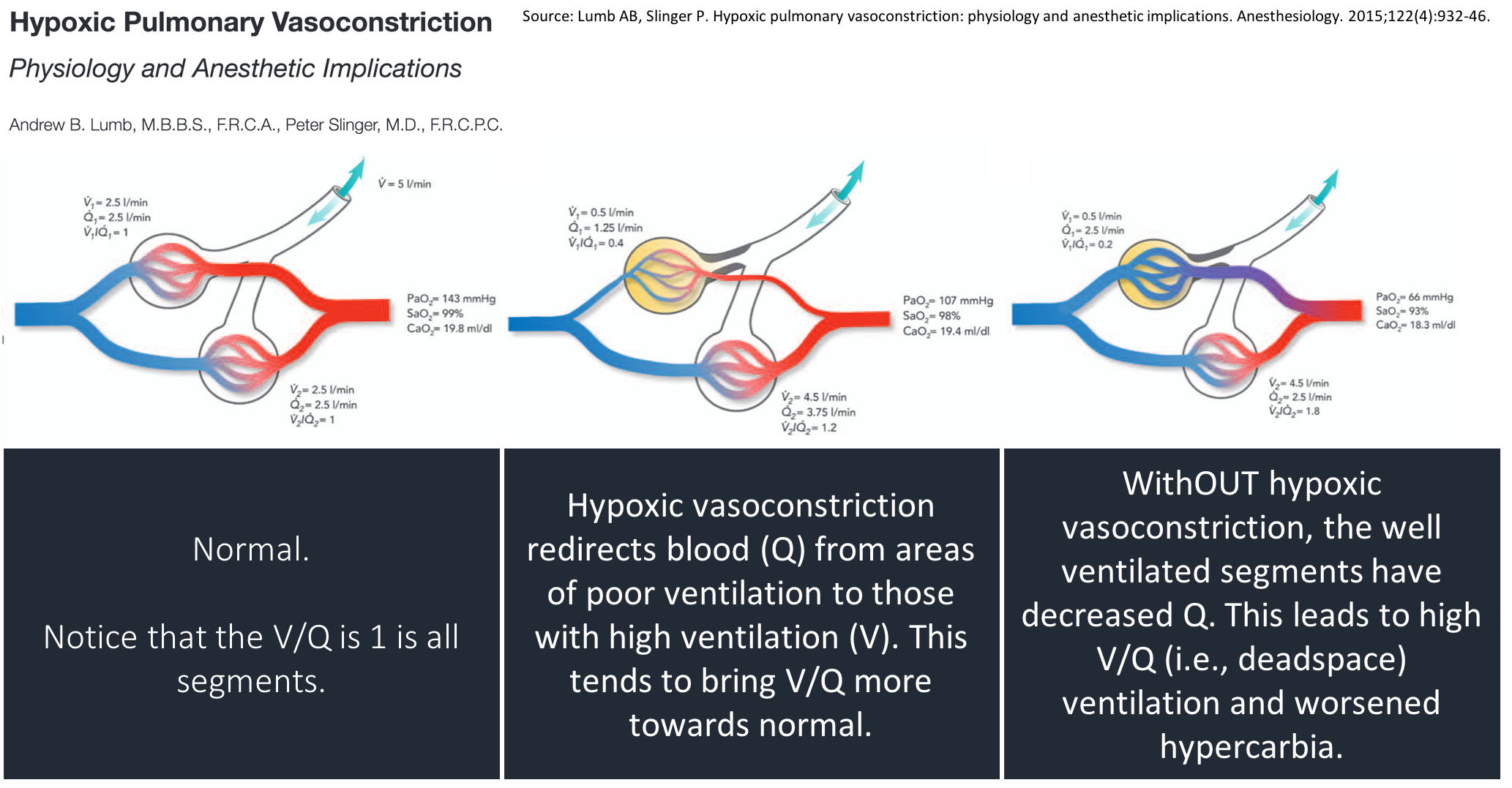
Some subsequent studies (e.g., PMID 9295826, PMID 8565533) have supported the theory that loss of hypoxic vasoconstriction & altered V/Q is the main driver of hypercarbia with the administration of O₂. But, not all agree (e.g., PMID 3590052).
This thread has addressed patients with acute COPD. Does the same apply to patients with stable COPD? One study examined stable COPD patients breathing 100% O₂ for 15min: Ventilation decreased by 0.08 L/min (this is tiny); pCO₂ increased by 6.6mmHg (also small). https://ncbi.nlm.nih.gov/pubmed/9032202
What explains the small change in ventilation and pCO₂ seen in stable COPD patients? One reason: the decrease in ventilation one might expect from the administration of 100% O₂ was "offset" by the appropriate increase in ventilation in response to an increase in pCO₂.
Compare this with patients with acute COPD. They are already breathing at or near their maximal sustainable ventilation. They are therefore are unable to augment their ventilation in response to an increase in pCO₂.
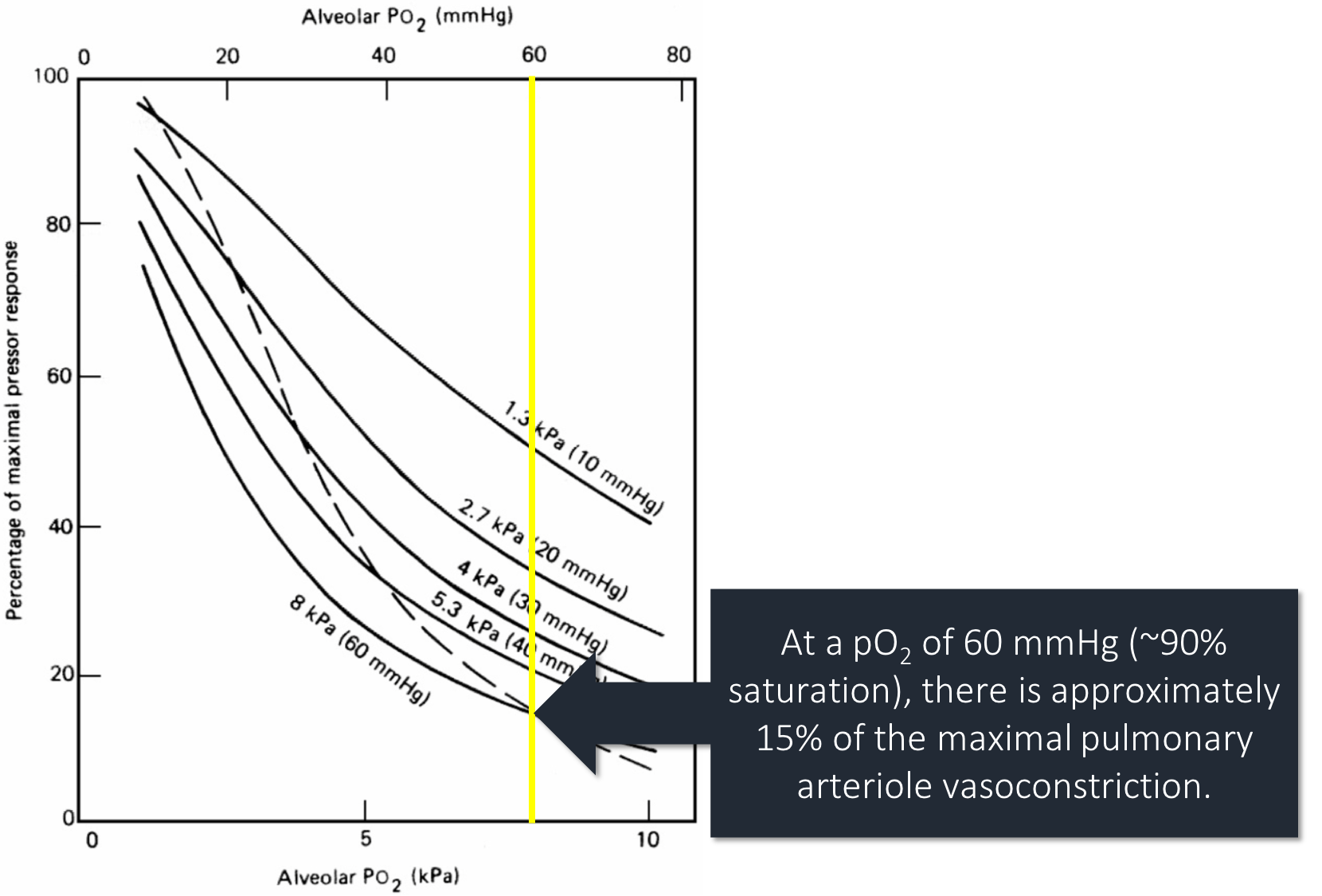
Another potential difference is the degree of hypoxia. At a pO₂ of 60mmHg vasoconstriction is 15% of the max. As pO₂ falls, vasoconstriction increases rapidly. Stable patients, with less severe hypoxia, may not have much vasoconstriction to "lose".
Regarding our goal SaO₂ of 88-92%, this partly comes from RCT data comparing O₂ titrated to this range versus high flow O₂. One study of 405 patients with acute COPD reported mortality of:
HR 0.48 (p=0.02) https://ncbi.nlm.nih.gov/pubmed/20959284
From the perspective of a cardiology fellow, it’s always V/Q mismatch when something is wrong with the lungs. Question is, is the mismatch high or low...
Source:
https://mobile.twitter.com/tony_breu/status/1070736103072784384
Alveolar Capillary Interface
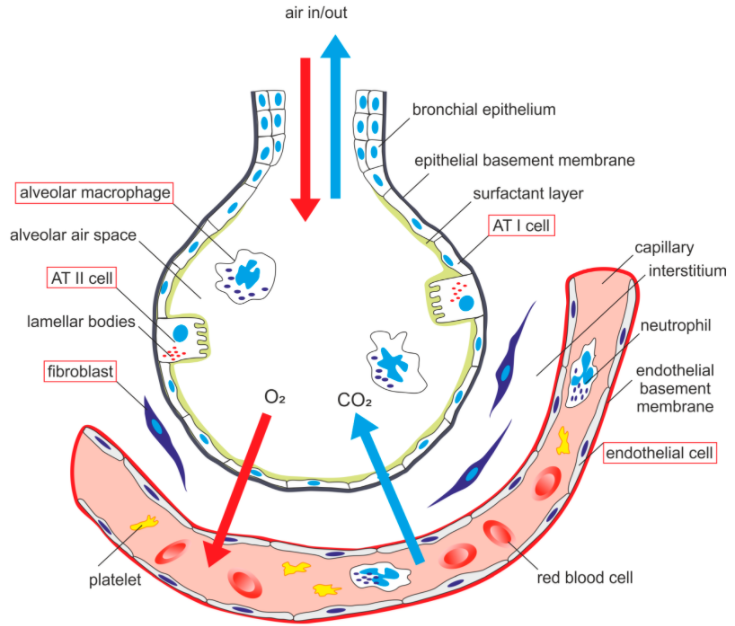
Dead space and shunt. Dead space refers to areas that are ventilated but not perfused, whereas shunt refers to areas that are perfused but not ventilated. V = ventilation; Q = blood flow.
Dead space: Ventilation but no perfusion
Types of dead space:
Anatomic dead space: Volume of the conducting airways (i.e., areas that move air but do not participate in gas exchange; ~150 mL)
Alveolar dead space: refers to the volume of air in alveoli that are ventilated but not perfused, and thus gas exchange does not take place.
Physiologic dead space: is composed of Anatomic dead space, Alveolar dead space, shunt. Functional measurement of the volume of the lungs that does not participate in gas exchange.
Calculating physiologic dead space: VD = VT × (PACO2 – PECO2)/(PACO2)
VD = Physiologic dead space, VT = Tidal volume, PACO2 = PCO2 of arterial blood = PCO2 of alveolar gas, PECO2 = PCO2 expired air
If physiologic dead space > predicted anatomic dead space, then pathology is present that increases dead space
- E.g., pulmonary embolism – clot disrupts blood flow (i.e., Q = 0) → V/Q = infinity; 100% O2 will help
- V/Q mismatch is more likely to cause hypoxemia than hypercapnia
O2 has a sigmoidal hemoglobin binding curve and thus is generally saturated in the alveolar–capillary bed (i.e., exchange only increases with increased blood flow) → hyperventilating does not help
CO2 has a linear hemoglobin binding curve, and increased ventilation can increase removal from blood → hyperventilating can help/compensate for mismatch
Oxygenation
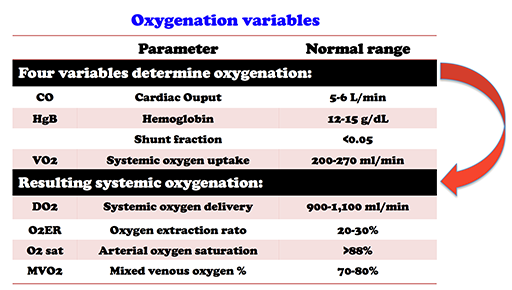
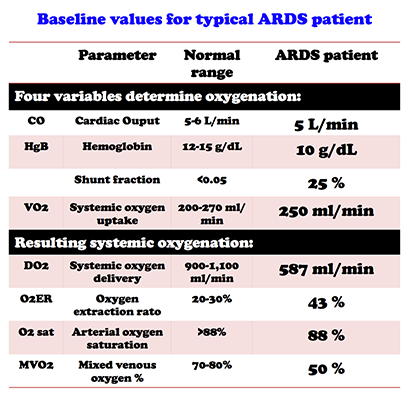
Source: https://emcrit.org/pulmcrit/ards-oxygen-extraction-ratio/
Transpulmonary Pressure = Transmural Pressure
From ChatGPT:
Transpulmonary pressure (TPP) is the difference between the pressure inside the alveoli (intra-alveolar pressure) and the pressure outside the lungs (pleural pressure). When TPP is zero, it means that the pressure inside the alveoli and the pressure outside the lungs are equal.
In the alveoli, when TPP is zero, there is no pressure gradient to drive airflow in or out of the lungs. Therefore, there is no movement of air in or out of the lungs. This is the end-expiratory position of the respiratory system, where the lungs are at their resting volume and the alveoli are filled with air. At this point, gas exchange between the alveoli and the pulmonary capillaries continues by diffusion, driven by differences in the partial pressures of oxygen and carbon dioxide across the alveolar-capillary membrane.
It is important to note that TPP is not always zero during normal breathing. TPP increases during inhalation and decreases during exhalation, creating a pressure gradient that drives airflow into and out of the lungs. Therefore, TPP of zero occurs only during the resting phase of breathing.
CO2
Carbon dioxide is produced during the process of cellular respiration in the mitochondria of the cell. More specifically, carbon dioxide is a byproduct of the Krebs cycle, which is also known as the citric acid cycle or the tricarboxylic acid (TCA) cycle. During the Krebs cycle, acetyl-CoA molecules derived from the breakdown of glucose and other energy sources are oxidized to produce ATP, NADH, and FADH2, which are used in the electron transport chain to generate more ATP. As a byproduct of the Krebs cycle, carbon dioxide is produced and released into the bloodstream. The carbon dioxide is then transported to the lungs, where it is exhaled out of the body.
Videos:
Meet Patel: LUNG AND CHEST WALL GRAPH | COMPLIANCE explained | USMLE STEP 1 | RESPIRATORY PHYSIOLOGY - YouTube https://www.youtube.com/watch?v=Lf8vs76uMAE&t=48s
The slope of the pressure-volume curve in pulmonary physiology represents dynamic compliance, which takes into account the resistance to airflow and the compliance of the lung and chest wall during movement or airflow.
Static compliance refers to the change in volume for a given change in pressure when the system is not moving or when airflow is completely halted. It is determined by the elastic properties of the lung and chest wall. Static compliance is calculated by dividing the change in volume by the change in pressure.
On the other hand, dynamic compliance refers to the change in volume for a given change in pressure during movement or airflow. It takes into account the resistance to airflow and the compliance of the lung and chest wall. The slope of the pressure-volume curve during inspiration or expiration when airflow is present represents dynamic compliance.
The pressure-volume curve is a graphical representation of the relationship between the pressure applied to the respiratory system and the resulting changes in lung volume. The slope of this curve at any given point represents the compliance of the system at that particular volume. Compliance is a measure of the distensibility or stiffness of the lungs, and it reflects how easily the lungs can expand or contract in response to changes in pressure.
Compliance depends on the initial lung volume from which the change in volume is measured and the ventilatory events immediately preceding the measurement as well as the properties of the lung itself. At large lung volumes, compliance is lower, because the lung is nearer its elastic limit. If the subject has breathed with a fixed tidal volume (Vt) for some minutes, portions of the lung are not participating in ventilation, and compliance may be reduced. A few deep breaths, with return to the initial volume, will increase compliance. Thus, a careful description of associated events is required for correct interpretation of the measurement.
Pressure-Volume Curves
The lower inflection point is thought to reflect the point at which small airways or alveoli reopen, corresponding to closing volume.
In patients with acute lung injury, investigators have recommended that PEEP be set at a pressure slightly above the lower inflection point.
The importance of individualizing PEEP in ventilator management in patients with ARDS was recently confirmed in a Spanish trial. One group of patients was randomized to a treatment group in which PEEP was titrated to 2 cm H2O above the lower inflection point. The mortality rate was lower in these patients than in control group patients who did not receive customized titration of PEEP.
In respiratory physiology, hysteresis is defined as the difference between the transpulmonary pressure of inhalation (increasing volume) and the pressure of exhalation (decreasing volume).
In healthy lung tissue, the elastic fibres of the surrounding alveoli pull on the walls of small airways and hold them open – this force is called radial traction. The higher the elastic recoil of the lungs, the greater the radial traction will be. Radial traction helps to prevent airway collapse in expiration.
Source: https://teachmephysiology.com/respiratory-system/ventilation/airway-resistance/#:~:text=In%20healthy%20lung%20tissue%2C%20the,prevent%20airway%20collapse%20in%20expiration.
The autonomic nervous system usually determines airway diameter. Sympathetic innervation causes relaxation of bronchial smooth muscle via beta-2 receptors, which causes an increase in airway diameter to allow more airflow. This is useful in situations such as exercise, when sympathetic stimulation triggers airway muscle relaxation to allow more air into the lungs.
Conversely, parasympathetic innervation works on muscarinic (M3) receptors to increase smooth muscle contraction and reduce diameter.
Source: https://teachmephysiology.com/respiratory-system/ventilation/airway-resistance/#:~:text=In%20healthy%20lung%20tissue%2C%20the,prevent%20airway%20collapse%20in%20expiration.
Lutfi article here